Introduction
Materials and Methods
Materials
Sample preparation
Cell culture and treatment
Cell viability assay
Measurement of NO production
Isolation of cytosol and nucleus fraction
SDS-PAGE and Western blot
Reverse transcriptase-polymerase chain reaction (RT-PCR)
Statistical analysis
Results and Discussion
The effect of the part of A. cordata on NO production in LPS-stimulated RAW264.7 cells
The effect of AC-L on NO production and iNOS, COX-2 and IL-1β expression in LPS-stimulated RAW264.7 cells
The effect of AC-L on NF-κB signaling activation in LPS-stimulated RAW264.7 cells
The effect of AC-L on MAPK signaling activation in LPS-stimulated RAW264.7 cells
Introduction
Inflammation is an innate and protective response mechanism of the immune system to resist foreign infection and tissue injury, and promote the restorable tissue structure and remove invading pathogens. Inflammation including chronic and acute inflammation presents double-sidedness. Inflammatory mediators-induced chronic inflammation is considered to be a cause of numerous human diseases including cancer, allergy, atherosclerosis, gastro-enteritis, arthritis and septic shock (Guzik et al., 2003; Ha et al., 2010; Chen et al., 2015). Prostaglandin E2 (PGE2) and nitric oxide (NO) are the principal chemical mediators of inflammation. Cyclooxygenase-2 (COX-2) is mainly responsible for the synthesis of significantly amounts of prostaglandins (PGs) in inflammatory disorders. In addition, the inducible nitric oxide synthase (iNOS) produces enormous amount of NO in several inflammatory disease (Nathan, 1992; Zarghi and Arfaei, 2011; Kim et al., 2018).
Aralia cordata (A. cordata) is a perennial herbaceous plant belonging to Araliaceae, It is widely distributed in mountainous regions of East Asia such as Japan and China. The root of A. cordata has reported to contains essential oil (limonen, alpha-pinene, gamma-terpinene, myrcene, humulene, alpha-pinene, caryophyllene, alpha-copaene and terpinene-4-ol), stearic acid, resin, salicylic acid and diterpenic acid I, II, copper, manganese and nickel (Lim et al., 2009; Kang et al., 2012). The methanol extracts from A. cordata has reported to inhibit IL-8 production by lipopolysaccharide (LPS)-treated peritoneal macrophages. Thus, there is a need for the development of anti-inflammatory agents that effectively and safely block and modulate excessive inflammatory responses. The aim of this study was to evaluate of stems, roots and leaves of A. cordata as an anti - inflammatory agent.
Materials and Methods
Materials
Dulbecco’s Modified Eagle medium (DMEM)/F-12 1:1 Modified medium (DMEM/F-12) was purchased from Lonza (Walkersville, MD, USA). LPS (Escherichia coli 055:B5) and 3-(4,5-dimethylthiazol-2-yl)-2.5-diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Antibodies against IκB-α, phospho-IκB-α, p65, phospho-ERK1/2, ERK1/2, phospho-p38, p38, and β-actin were purchased from Cell Signaling (Bervely, MA, USA).
Sample preparation
The stems (AC-S), roots (AC-R) and leaves (AC-L) of Aralia cordata (A. cordata) were collected in the september 2018 from Hancheon-ri, Bonghyeon-myeon, Punggi-eup, Youngju-si, Korea. 10g of dried AC-S, AC-R and AC-L of A. cordata were extracted with 200㎖ of 100% methanol for 24 hours under shaking at the room temperature. After 24 hours, the methanol-soluble fraction was filtered and concentrated a vacuum evaporator(EYELA, Rotary evaporator N-1000, Japan). The methanol extracts from the AC-S, AC-R and AC-L were kept in a refrigerator until use.
Cell culture and treatment
Mouse macrophage cell line, RAW264.7 was purchased from Korean Cell Line Bank (Seoul, Korea) and grown in DMEM/F-12 supplemented with 10% fatal bovine serum (FBS), 100 U/㎖ penicillin and 100 ㎍/㎖ streptomycin. The cells were maintained at 37℃ under a humidified atmosphere of 5% CO2. AC-S, AC-R and AC-L were dissolved in dimethyl sulfoxide (DMSO) and treated to cells. DMSO was used as a vehicle and the final DMSO concentration did not exceed 0.1% (v/v).
Cell viability assay
Cell viability was evaluated by MTT assay. Briefly, cells were plated at a density of 3 × 104 cells/well in 12-well plate and incubated for 24 hours. The cells were treated with AC-S, AC-R and AC-L at the indicated concentrations for 24 hours. Then, the cells were incubated with 200 ㎕ of MTT solution (1 ㎎/㎖) for an additional 2 hours. The resulting crystals were dissolved in DMSO. The formation of formazan was measured by reading absorbance at a wavelength of 570 ㎚ using UV/Visible spectrophotometer (Tecan infinite M200pro, Switzerland).
Measurement of NO production
RAW264.7 cells were plated in 12-well plate for overnight. The cells were pretreated with AC-S, AC-R and AC-L at the indicated concentrations for 6 hours and then co-treated with LPS (1㎍/㎖) for 18 hours. NO levels were measured by Griess assay. Briefly, 50 ㎕ of the cell culture supernatants were mixed with 50 ㎕ of Griess reagent (Sigma Aldrich, St. Louis, MO, USA) and followed by incubation for 15 minutes at the room temperature. After 15 minutes, absorbance values were measured using a UV/Visible spectrophotometer (Tecan infinite M200pro, Switzerland) at 540 ㎚.
Isolation of cytosol and nucleus fraction
Cytosol and nuclear fractions of cells were prepared using a nuclear extract kit (Active Motif, Carlsbad, CA, USA) according to the manufacturer's protocols. Briefly, RAW264.7 cells were washed with ice-cold 1×phosphate-buffered saline (PBS) containing phosphatase inhibitors and harvested with 1xhypotonic buffer for 15 minutes at 4℃. After adding detergent and vortexing for 10 seconds, the cells were centrifuged at 14,000 g for 1 minute at 4℃ and the supernatants (cytoplasmic fraction) were collected and stored at -80℃ for further analysis. The cell pellets were used for nuclear fraction collection. Cell pellets were re-suspended with lysis buffer by pipetting up and down, and incubated at 4℃ for 30 minutes under shaking. After 30 minutes, nuclear suspensions were centrifuged at 14,000 g for 10 minutes at 4℃, and the supernatants (nuclear fraction) were stored at -80℃ for further analysis.
SDS-PAGE and Western blot
Cells were washed with 1×PBS, and lysed in radioimmunoprecipitation assay (RIPA) buffer (Boston Bio Products, Ashland, MA, USA) supplemented with protease inhibitor cocktail (Sigma Aldrich) and phosphatase inhibitor cocktail (Sigma Aldrich), and centrifuged at 15,000 rpm for 10 minutes at 4℃. Protein concentration was determined by the bicinchoninic acid (BCA) protein assay (Pierce, Rockford, IL, USA) using bovine serum albumin (BSA) as the standard. The proteins were separated on SDS-PAGE and transferred to nitrocellulos membrane (GE healthcaer life sciences). The membranes were blocked for non-specific binding with 5% skim milk in Tris-buffered saline containing 0.05% Tween 20 (TBS-T) for 1hour at room temperature and then incubated with specific primary antibodies in 5% skim milk at 4℃ overnight. After three washes with TBS-T, the blots were incubated with horse radish peroxidase (HRP)-conjugated immunoglobulin G (IgG) for 1 hour at room temperature and chemiluminescence was detected with ECL Western blotting substrate (Amersham Biosciences) and visualized in chemi doc (Bio-rad, Chemi Doc MP Imaging system, USA).
Reverse transcriptase-polymerase chain reaction (RT-PCR)
Total RNA was prepared using a RNeasy Mini Kit (Qiagen, Valencia, CA, USA) and total RNA (1 ㎍) was reverse-transcribed using a Verso cDNA Kit (Thermo Scientific, Pittsburgh, PA, USA) according to the manufacturer's protocol for cDNA synthesis. PCR was carried out using PCR Master Mix Kit (Promega, Madison, WI, USA) with primers for mouse iNOS, mouse COX-2, mouse IL-1β and mouse GAPDH as follows:
iNOS Forward 5′-GTGCTGCCTCTGGTCTTGCAAGC-3′
iNOS Reverse 5′-AGGGGCAGGCTGGGAATTCG-3′
COX-2 Forward 5′-GGAGAGACTATCAAGATAGTGATC-3′
COX-2 Reverse 5′-ATGGTCAGTAGACTTTTACAGCTC-3′
IL-1β-Forward 5′-GAAGCTGTGGCAGCTACCTATGTCT-3′
IL-1β-Reverse 5′-CTCTGCTTGTGAGGTGCTGATGTAC-3′
GAPDH Forward 5′-CAGGAGCGAGACCCCACTAACAT-3′
GAPDH Reverse 5′-GTCAGATCCACGACGGACACATT-3′
Statistical analysis
All the data are shown as mean ± SD (standard deviation). Statistical analysis was performed with one-way ANOVA followed by Dunnett's test. Differences with *P or #P < 0.05 were considered statistically significant.
Results and Discussion
The effect of the part of A. cordata on NO production in LPS-stimulated RAW264.7 cells
Mouse macrophages play an important role in inflammatory response by producing inflammatory mediators such as NO and IL-1β (Nathan, 1992; Bogdan, 2015). So, we used the mouse macrophage cell line RAW264.7 cells for evaluating anti-inflammatory effect of part of A. cordata. To determine if the parts of A. cordata could reduce NO generation by LPS, RAW264.7 cells were pretreated with the extracts from the parts of A. cordata for 6 hours and then co-treated with LPS (1 ㎍/㎖) for the additional 18 hours. As shown in Fig. 1A, treatment of LPS without A. cordata induced NO overproduction in LPS-stimulated RAW264.7 cells, while pretreatment of all A. cordata blocked LPS mediated NO overproduction. Among A. cordata, the inhibitory effect of NO production was highest in the treatment of AC-L. Thus, we chose AC-L for further studies. We evaluated the effect of AC-L on the viability of RAW264.7 cells. As shown in Fig. 1B, AC-L maintained the cell viability.
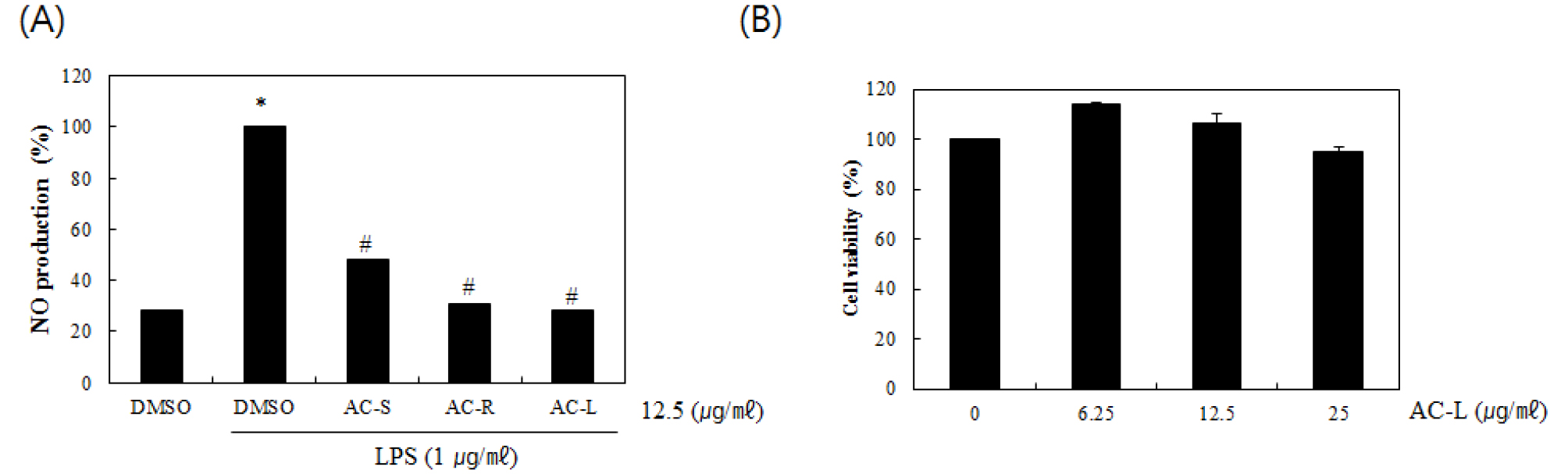
Fig. 1.
Effect of the part of A. cordata on NO production in LPS-stimulated RAW264.7 cells. (A) RAW264.7 cells were pretreated with AC-S, AC-R and AC-L for 6 hours and then co-treated with LPS (1 ㎍/㎖) for 18 hours. The determination of NO production was measured by Griess assay. (B) RAW264.7 cells were treated with AC-L at the indicated concentrations for 24 hours. Cell viability was measured using MTT assay system and expressed as % cell viability. *P < 0.05 compared to the cells without the treatment, and #P < 0.05 compared to the cells treated with LPS alone.
The effect of AC-L on NO production and iNOS, COX-2 and IL-1β expression in LPS-stimulated RAW264.7 cells
NO is a key target for managing inflammatory diseases. NO is synthesized by nitric oxide synthases (NOSs) from the amino acid L-arginine. Among three types NOS isoforms including neuronal NOS (nNOS), endothelial NOS (eNOS) and inducible NOS (iNOS), only iNOS is overexpressed in LPS-stimulated mouse macrophage and subsequently generates NO. COX-2 expression is quickly induced by stimuli such as proinflammatory cytokines (IL-1β), inflammation and oncogenes. Thus, the inducible isozyme has been involved in pathological processes such as various cancer types and inflammation. IL-1β is a one of the interleukin 1 family of cytokines. This cytokine is an key mediator of the inflammatory response, and is involved in a variety of cellular activities, including apoptosis, differentiation and cell proliferation (Tsuzaki et al., 2003; Wang and Dubois, 2010 ; Zarghi and Arfaei, 2011; Park et al., 2014).
Since NO production is regulated by iNOS, COX-2 and IL-1β expression, the effect of AC-L on iNOS, COX-2 and IL-1β expression was evaluated by Western blot and RT-PCR. As shown in Fig. 2A, treatment of LPS without AC-L induced NO overproduction in LPS-stimulated RAW264.7 cells, while pretreatment of all AC-L inhibited LPS mediated NO overproduction. As shown in Fig. 2B, LPS overexpression was detected in the cells stimulated LPS alone. However, AC-L suppressed iNOS and COX-2 expression in LPS-stimulated RAW264.7 cells. For the further study for evaluating whether inhibitory effect of AC-L against the expression of iNOS and COX2 protein is mediated from regulating iNOS and COX2 transcription, we also determined the mRNA level of iNOS and COX2 regulated by AC-L in LPS-stimulated RAW264.7 cells. As shown in Fig. 2C, the treatment of AC-L attenuated the mRNA expression of iNOS, COX-2 and IL-1β. Thus, AC-L attenuated the over-production of NO by suppressing LPS-induced iNOS, COX-2 and IL-1β over-expression in RAW264.7 cells.
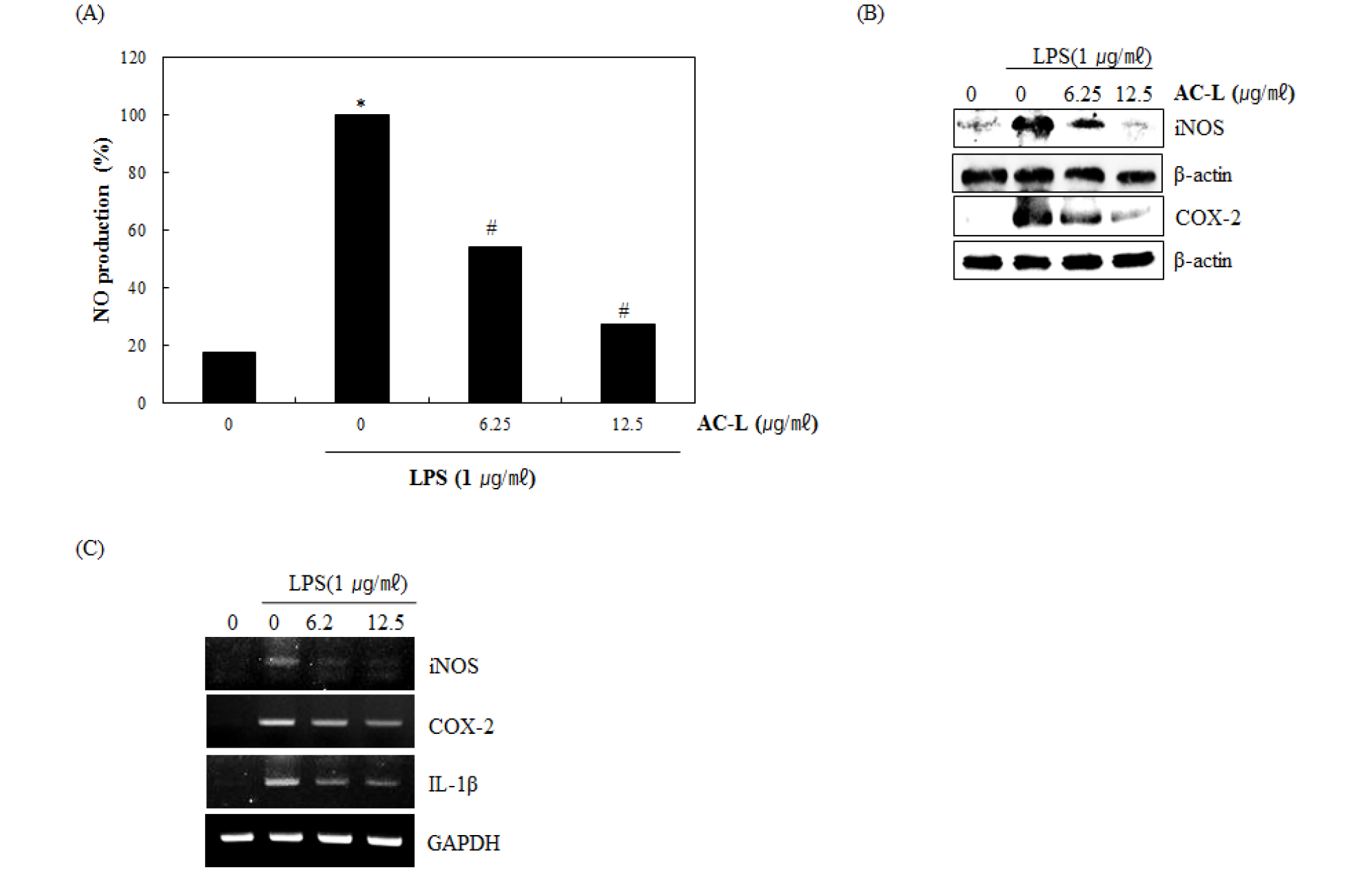
Fig. 2.
The effect of AC-L on NO production and iNOS, COX-2 and IL-1β expression in LPS-stimulated RAW264.7 cells. (A) RAW264.7 cells were pre-treated with AC-L at the indicated concentrations for 6 hours and then co-treated with LPS (1 ㎍/㎖) for the additional 18 hours. After treatment, NO production was measured using the media and Griess reagent and (B) cell lysates were resolved by SDS-PAGE, transferred to nitrocellulose membrane, and probed with iNOS and COX-2 antibody for Western blot. iNOS and COX-2 protein was visualized using ECL was used as a vehicle. (C) For RT-PCR, RAW264.7 cells were pre-treated with AC-L at the indicated concentrations for 6 hours and then co-treated with LPS (1 ㎍/㎖) for the additional 18 hours. Total RNA was isolated and RT-PCR was performed for iNOS and COX-2 and IL-1â. Values given are the mean ± SD (n = 3). *P < 0.05 compared to LPS treatment without AC-L. GAPDH was used as an internal control for RT-PCR.
The effect of AC-L on NF-κB signaling activation in LPS-stimulated RAW264.7 cells
Nuclear factor-κB (NF-κB) and its role in inflammatory responses, immune reactions, and tumorigenesis has been widely studied. NF-κB activation induces various target genes, such as cell survival, angiogenesis, proliferation, tumor promotion and inflammation. Regulation and activation of NF-κB are tightly controlled by IκB proteins. IκBα is one of the group of cellular proteins that function to suppress the NF-κB transcription factor (Verma et al., 1995; Nishikori, 2005; Lawrence, 2009; Gantke, 2012; Taniguchi and Karin, 2018). To elucidate the effect of AC-L on NF-κB signaling activation, we examined a western blot for IκB-α degradation in LPS-stimulated RAW264.7 cells. As shown in Fig. 3A, LPS induced subsequent IκB-α degradation and IκB-α phosphorylation at 30 minutes after the stimulation. However, pretreatment of AC-L inhibited LPS-induced IκB-α degradation in a dose-dependent manner. p65 translocation from cytosol to nucleus resulted from IκB-α degradation by various stimuli are essential in NF-κB activation. Thus, we performed whether AC-L suppresses nuclear translocation of p65. As shown in Fig. 3B, LPS increased an amount of p65 in the nucleus of RAW264.7 cells. However, pretreatment of AC-L dose-dependently blocked LPS-induced p65 translocation. Translocate p65 into the nucleus binds to the NF-κB binding site and increases NF-κB transcriptional activity. These data show that AC-L may block NF-κB activation by suppression of p65 translocation into the nucleus via inhibiting the IκB-α degradation.
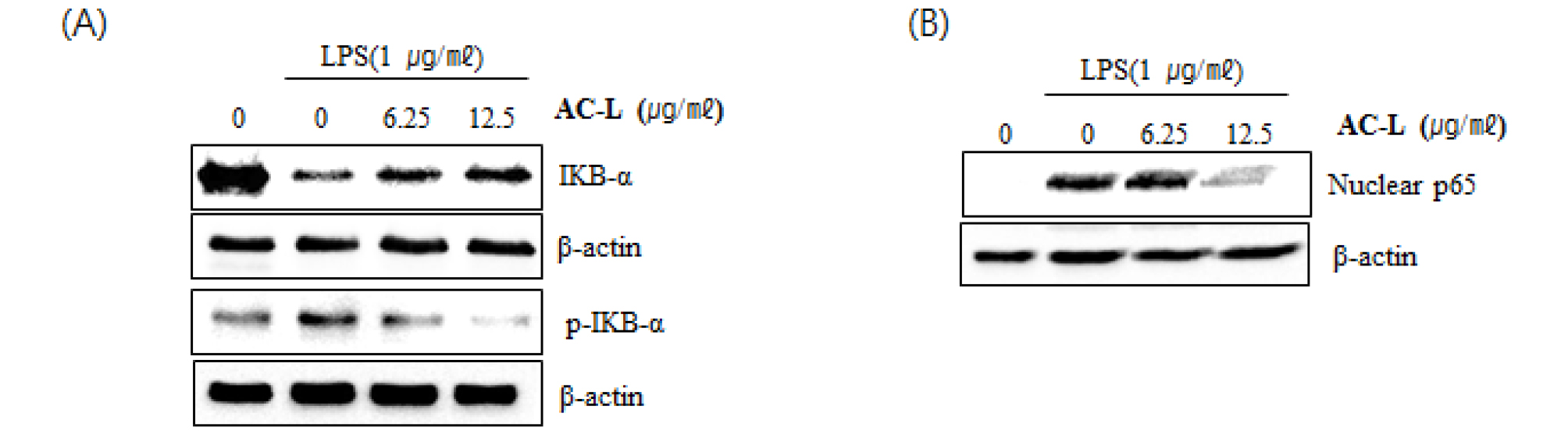
Fig. 3.
The effect of AC-L on NF-κB signaling activation in LPS-stimulated RAW264.7 cells. (A) RAW264.7 cells were pretreated with AC-L for 6 hours and then co-treated with LPS (1 ㎍/㎖) for 20 minutes. (B) RAW264.7 cells were pretreated with AC-L for 6 hours and then co-treated with LPS (1 ㎍/㎖) for 30 minutes. After the treatment, the cytosol and nucleus were prepared. For Western blot analysis, the cell lysates were subjected to SDS-PAGE and the Western blot was performed using antibodies against IκB-α and p65. Actin was used as internal control.
The effect of AC-L on MAPK signaling activation in LPS-stimulated RAW264.7 cells
Mitogen-activated protein kinases (MAPKs) are involved in the production of the inflammatory mediators. There are three main components of the MAPK family, including the the p38 MAPK, the c-Jun N-terminal kinases (JNKs) and extracellular signal-regulated kinases (ERKs). In addition, MAPK have been reported to activate NF-κB pathway. The inflammatory stimuli induce MAPK activation by phosphorylating ERK1/2, p38 and JNKs, which results in the expression of the inflammatory mediators such as iNOS and IL-1β (Surh et al., 2001; Dolcet et al., 2005; Gloire et al., 2006; Sun et al., 2013). Thus, MAPK has been regarded as the potential target for the development of anti-inflammatory agents. To further investigate whether decrease of NF-κB activation by AC-L treatment is associated with the modulation of ERK1/2 and p38 activation, we evaluated the effects of AC-L on phosphorylation of ERK1/2 and p38 in LPS-stimulated RAW264.7 cells. As shown in Fig. 4, increase of ERK1/2 and p38 phosphorylation was observed in RAW264.7 cells by LPS. However, AC-L suppressed phosphorylation of ERK1/2 and p38, indicating that AC-L blocks the inflammatory response by inhibiting ERK1/2 and p38 activation in LPS-stimulated RAW264.7 cells.
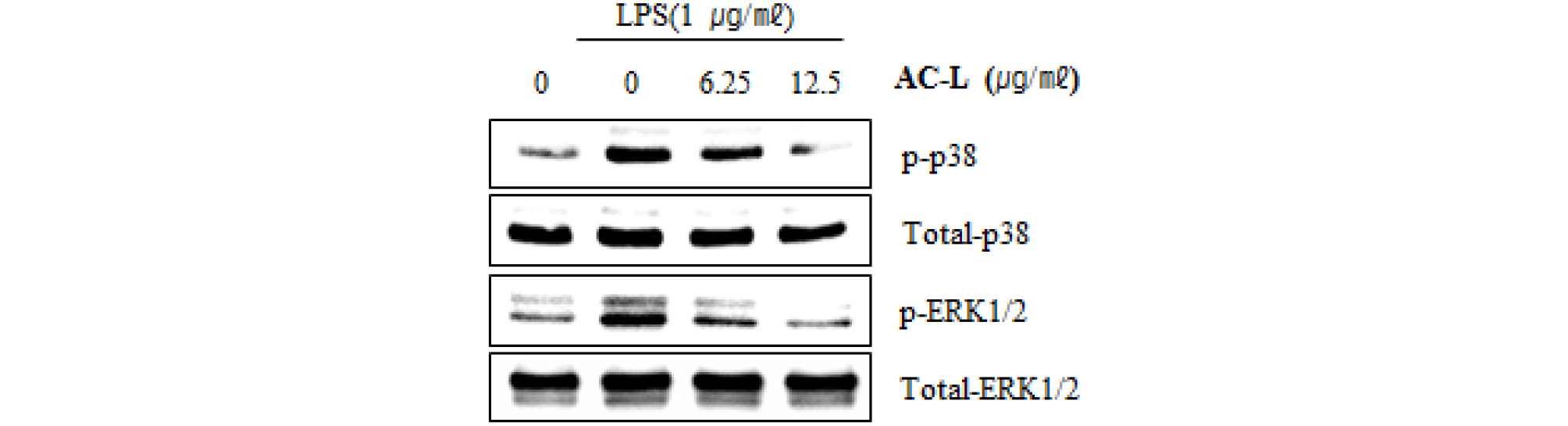
Fig. 4.
The effect of AC-L on MAPK signaling activation in LPS-stimulated RAW264.7 cells. RAW264.7 cells were pretreated with AC-L for 6 hours and then co-treated with LPS (1 ㎍/㎖) for 30 minutes. For Western blot analysis, the cell lysates were subjected to SDS-PAGE and the Western blot was performed using antibodies against p-ERK1/2, p-p38 and Total p-38 and Total ERK1/2 were used as internal control.
Taken together, these results demonstrated that AC-L blocked NO production via inhibiting iNOS, COX-2 and IL-1β expression through suppressing the activation of NF-κB and MAPK. The anti-inflammatory effect and potential mechanisms of AC-L will provide the potential usage of AC-L in the anti-inflammatory drug development.




