Introduction
Materials and Methods
Plant materials and reagents
Callus induction
Extraction and fractionation
Identification of acteoside by HPLC-PDA analysis
DPPH radical scavenging activity
ABTS radical scavenging activity
Inhibitory effect of φX-174 RF I plasmid DNA on oxidative DNA damage
Cell culture
SDS-PAGE and western blot analysis
Reverse transcriptase-polymerase chain reaction (RT-PCR)
Statistical analysis
Results and Discussion
Identification of acteoside from ECA
Antioxidant activities of ECA
Inhibitory effect of ECA against DNA cleavage by oxidative stress
Effect of ECA on DNA damage in NIH/3T3 cells induced by oxidative damage
Introduction
In the search for alternatives to selectively produce valuable compounds from plants, biotechnological approaches, particularly plant tissue cultures, have shown potential in the industrial production of bioactive plant metabolites (Giri and Narasu, 2000; Ramachandra and Ravishankar, 2002). Plants and cultured cells metabolize compounds in a qualitatively similar manner (Hellwig et al., 2004). Plant cell culture provides an attractive alternative source for extracting useful compounds from natural plant resources (Khanpour-Ardestani et al., 2015).
Oxidative stress is associated with the pathogenesis of some diseases such as cancer, inflammation, and heart disease, and DNA damage (Gilgun-Sherki et al., 2002). Additionally, reactive oxygen species (ROS)-mediated DNA damage regulates cell death and survival by modifying histone H2AX (Cook et al., 2009; Fernandez-Capetillo et al., 2004; Hamanaka and Chandel, 2010; Simon et al., 2000; Stucki et al., 2005). The tumor suppressor p53 has been demonstrated to regulate basal and ROS levels, which induce DNA damage (Liu et al., 2008; Polyak et al., 1997; Sablina et al., 2005). Thus, it is important for organisms to suppress the excessive generation of ROS. Antioxidants can remove ROS effectively through the oxidation-reduction reactions (Ji et al., 2015).
Acteoside was reported to have a variety of pharmacological properties such as anti-hepatotoxic (Xiong et al., 1998), anti-inflammatory (Schlesier et al., 2002), and antinociceptive effects (Xie and Wu, 1993) and antioxidant activity (He et al., 2000; Li et al., 1993; Xiong et al., 1996). Acteoside is generally present only in low amounts in plants (Imakura et al., 1985; Kitagawa et al., 1988).
Abeliophyllum distichum Nakai is a deciduous shrub of flowering plant in Oleaceae and has been regarded as an important plant resource because there is only one species in the world (Kang et al., 2000). A. distichum contains glycosides such as acteoside, isoacteoside, rutin, and hirsutrin (Oh et al., 2003). Although studies of the antioxidant (Ahn and Park, 2013; Park, 2011), anti-inflammatory (Park et al., 2014), and anti-cancer (Park et al., 2015) effects of A. distichum extract have been conducted, few studies have examined the biological usefulness of callus extracts. Thus, we investigated the antioxidant activity and inhibitory effects on oxidative DNA damage of extracts from the callus of A. distichum containing acteoside.
Materials and Methods
Plant materials and reagents
A. distichum Nakai (voucher number: Park1001(ANH)) was collected from Misun-hyang Theme park, Seongbul-Mountain Recreation Forest, 78, Chungmin-rogigok-gil, Goesan-eup, Goesan-gun, Chungcheongbuk-do, Korea. Methanol, petroleum ether, ethyl acetate, chloroform, acetonitrile, and dimethyl sulfoxide (HPLC-grade) were purchased from Merck (Darmstadt, Germany). Dulbecco’s modified Eagle’s medium (DMEM), 10% (v/v) fetal bovine serum, penicillin/streptomycin, and trypsin were purchased from Hyclone (Logan, UT, USA). The primary and secondary antibodies were purchased from Abcam (Cambridge, UK) and Santa Cruz Biotechnology (Dallas, TX, USA). All electrophoresis chemicals were purchased from Bio-Rad Labs (Hercules, CA, USA). All chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA), unless otherwise specified. Standard acteoside was purchased from Sigma-Aldrich.
Callus induction
To induce callus formation (Koh et al., 1989), 1-㎠ pieces of leaf explants were isolated from fresh plants. The explants were placed on MS medium (containing 4% sucrose and 0.9% agar, pH 5.7) supplemented with NAA and 2,4-D and cultured at 25℃ for 20 days to induce callus. Subsequently, a sufficient amount of callus was obtained through subculture in the same medium.
Extraction and fractionation
Callus from A. distichum was extracted with 80% methanol using a sonicator for 3 days. The methanol-soluble extracts were filtered and concentrated by using a vacuum evaporator (N-1110S, EYELA, Shanghai, China). The methanol-soluble extracts were fractionated using petroleum ether and ethyl acetate three times. Ethyl acetate fraction of callus from A. distichum (ECA) was acquired and stored in a refrigerator until use.
Identification of acteoside by HPLC-PDA analysis
A Waters 2695 system (Milford, MA, USA) equipped with Waters 2996 Photodiode array detector (PDA) was used to analyze ECA and the standard acteoside. Separation was carried out on an Xbridge-C18 (250 × 4.6 ㎜, 5 ㎛) with a C18 guard column. The binary mobile phase consisted of acetonitrile (solvent A) and water containing 1% acetic acid (solvent B). All solvents were filtered through a 0.45 ㎛ filter prior to use. The flow-rate was kept constant at 1.0 ㎖/min for a total run time of 20 min. The system was run with a gradient program: 0–20 min: 90% B to 50% B. The sample injection volume was 10 ㎕. Peaks of interest were monitored at 200–400 ㎚ by a PDA detector and compared with the standard acteoside.
DPPH radical scavenging activity
DPPH radical scavenging activity was measured according to Bondet method (Bondet et al., 1997) with some modifications. DPPH solution containing 300 µM 1,1-diphenyl-2-picryl hydrazyl (DPPH) in 95% alcohol was prepared. The solution was adjusted to an absorbance value of 1.00 at 515 ㎚. Next, 40 ㎕ of sample (0.32, 1.6, 8, 40, 200 ㎍/㎖) and 760 ㎕ of DPPH solution were mixed and incubated for 20 min in the dark at room temperature. Absorbance was measured using a UV/Visible spectrophotometer (Xma-3000PC, HumanCorp, Seoul, Korea) at 515 ㎚. DPPH radical scavenging activity was calculated according to the following equation:
DPPH radical scavenging activity (%) = [1 − (ASample − ABlank)/ACcontrol] × 100
ASample = Absorbance values of DPPH radicals after treatment with sample.
ABlank = Absorbance values of DPPH radicals with ethanol.
AControl = Absorbance values of DPPH radicals.
ABTS radical scavenging activity
ABTS radical scavenging activity was measured as described by Van den Berg et al. (1999) with some modifications. ABTS solutions containing 7.4 mM 2,2'-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt and 2.6 mM potassium persulfate in distilled water were prepared 24 h prior to the experiment. The solution was adjusted to an absorbance value of 0.70 at 734 ㎚. Next, 40 ㎕ of sample (0.32, 1.6, 8, 40, 200 ㎍/㎖) and 760 ㎕ of ABTS solution were mixed and incubated for 20 min in the dark at room temperature. Absorbance was measured using a UV/Visible spectrophotometer at 732 ㎚. ABTS radical scavenging activity was calculated according to the following equation:
ABTS radical scavenging activity (%) = [1 − (ASample − ABlank)/ACcontrol] × 100
ASample =Absorbance values of ABTS radicals after treatment with sample.
ABlank = Absorbance values of ABTS radicals with ethanol.
AControl = Absorbance values of ABTS radicals.
Inhibitory effect of φX-174 RF I plasmid DNA on oxidative DNA damage
Inhibition of oxidative DNA damage was measured as described by Jung and Surh (2001). Various concentrations (25, 50, and 100 ㎍/㎖) of ECA were pre-reacted with FeCl2 or FeSO4 and H2O2 for 15 min at 37℃. After the reaction, plasmid DNA was added to the mixtures and incubated at 37℃ for 1 min. After 1 min, 5 ㎕ of solution containing 50% glycerol (v/v), 40 mM EDTA, and 0.05% bromophenol blue was added to stop the reaction, and the reaction mixtures were electrophoresed on a 1% agarose gel with DNA SafeStain (Lamda Biotech, Ballwin, MO, USA). DNA in the gel was visualized and photographed using a FluorChem E (Cell Biosciences, Santa Clara, CA, USA).
Cell culture
The mouse skin fibroblast cell line NIH/3T3 was purchased from American Type Culture Collection (ATCC, Manassas, VA, USA) and grown in DMEM supplemented with 10% (v/v) fetal bovine serum, 100 U/㎖ penicillin, and 100 ㎍/㎖ streptomycin. The cells were maintained at 37℃ under a humidified atmosphere of 5% CO2. ECA was dissolved in DMSO and used to treat the cells. DMSO was used as a vehicle and the final DMSO concentration did not exceed 0.1% (v/v).
SDS-PAGE and western blot analysis
NIH/3T3 cells were cultured in 6-well plates at 37℃ in an incubator with a humidified atmosphere of 5% CO2. The cells were washed with 1× phosphate-buffered saline and lysed in radio immuno precipitation assay buffer (Thermo Scientific, Waltham, MA, USA) supplemented with protease inhibitor cocktail (Sigma-Aldrich), and then centrifuged at 12,000 ×g for 15 min at 4℃. The protein concentration of the sample was determined by the Bradford protein assay (Bio-Rad, Hercules, CA, USA). The proteins were mixed with Laemmli buffer and boiled at 95℃ for 5 min. The proteins were separated by 10% SDS-PAGE and transferred to a polyvinylidene fluoride membrane (Bio-Rad). The membranes were blocked for non-specific binding with 5% nonfat dry milk in Tris-buffered saline containing 1% Tween 20 (TBS-T) for 30 min at room temperature and then incubated with specific primary antibodies in 3% nonfat dry milk at 4℃ overnight. After washing three times with TBS-T, the blots were incubated with horseradish peroxidase-conjugated immunoglobulin G for 1 h at room temperature and chemiluminescence was detected using ECL Western blotting substrate (Bio-Rad) and visualized with FluorChem E (Cell Biosciences).
Reverse transcriptase-polymerase chain reaction (RT-PCR)
Total RNA was prepared from ECA treated-cells using a NucleoSpin® RNA Plus (Macherey-Nagel, Düren, Germany) and total RNA (1 ㎍) was synthesized using ReverTra Ace –α- (Toyobo, Osaka, Japan) according to the manufacturer’s protocol for cDNA synthesis. PCR was carried out using Quick Taq® HS DyeMix (Toyobo) with primers for H2AX (Forward: TTGCTTCAGCTTGGTGCTTAG, Reverse: AACTGGTATGAGGCCAGCAAC), p53 (Forward: CGGATAGTATTTCACCCTCAAGATCCG, Reverse: AGCCCTGCTGTCTCCAGACTC) and GAPDH (Forward: AACTTTGGCATTGTGGAAGG, Reverse: ATGCAGGGATGATGTTCTGG).
Statistical analysis
Statistical analysis was carried out using SPSS 18.0 (SPSS, Inc., Chicago, IL, USA). All experiments were performed at least three times. The data are shown as the mean ± SD of triplicate experiments. Differences were considered statistically significant when the p value was < 0.05.
Results and Discussion
Identification of acteoside from ECA
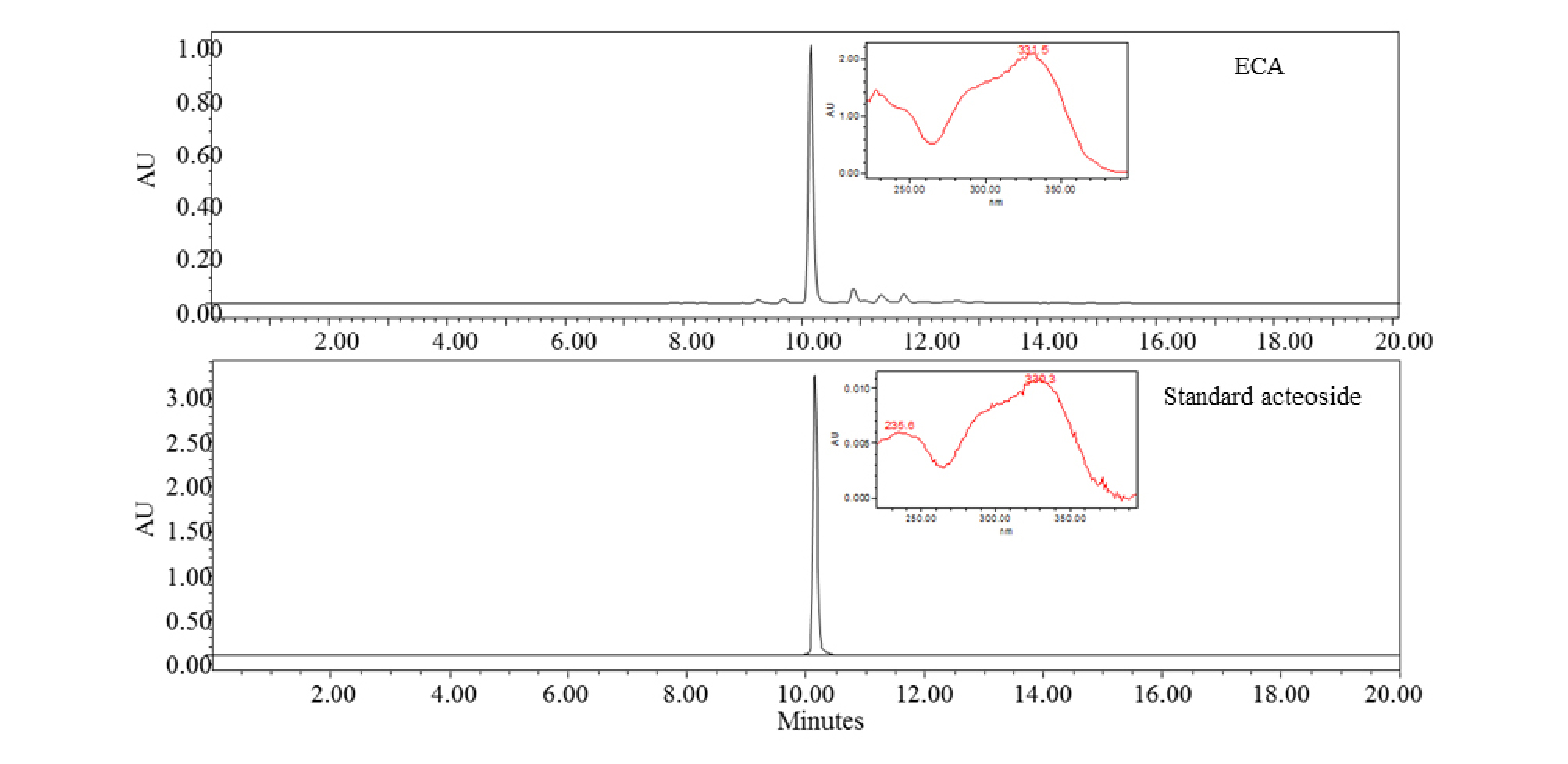
According to Bremer et al. (2002), many plants in the Oleaceae family such as Abeliophyllum, Forsythia, and Jasminum Genus contain acteoside. Its potential bioactivities have remarkable value in the industry and research fields. ECA was analyzed by HPLC-PDA. Fig. 1 shows the HPLC-PDA chromatogram and spectrum of ECA and standard acteoside. ECA and standard acteoside showed the same retention times (10.4 min) and UV spectra. Therefore, ECA was identified as an acteoside based on the HPLC-PDA data. The quantities of acteoside were 0.39 ㎎/g according to comparison with the standard curve.
Antioxidant activities of ECA
Antioxidants suppress ROS generated during intracellular metabolism. Our group reported that A. distichum leaves (Park, 2011), flowers (Ahn and Park, 2013), and immature seeds (Jang and Park, 2017) have strong antioxidant and other biological activities, but the antioxidant activity of callus has not been reported. To evaluate the antioxidant activity of callus from A. distichum, the ethyl acetate fraction was obtained from callus and the DPPH and ABTS scavenging activities were measured.
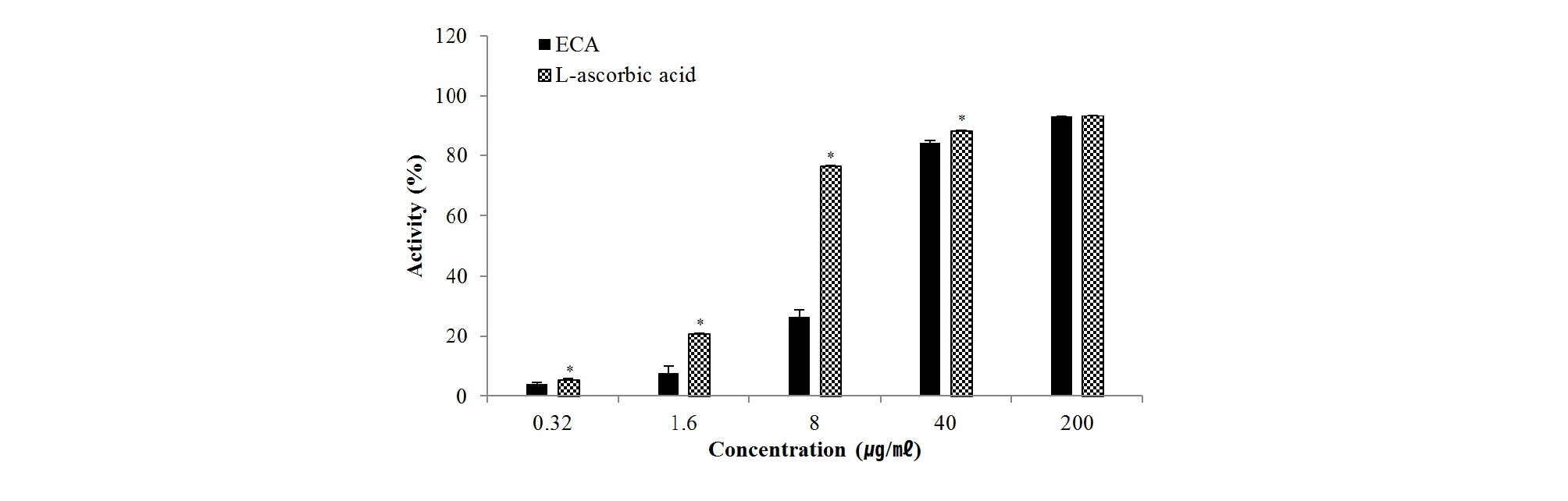
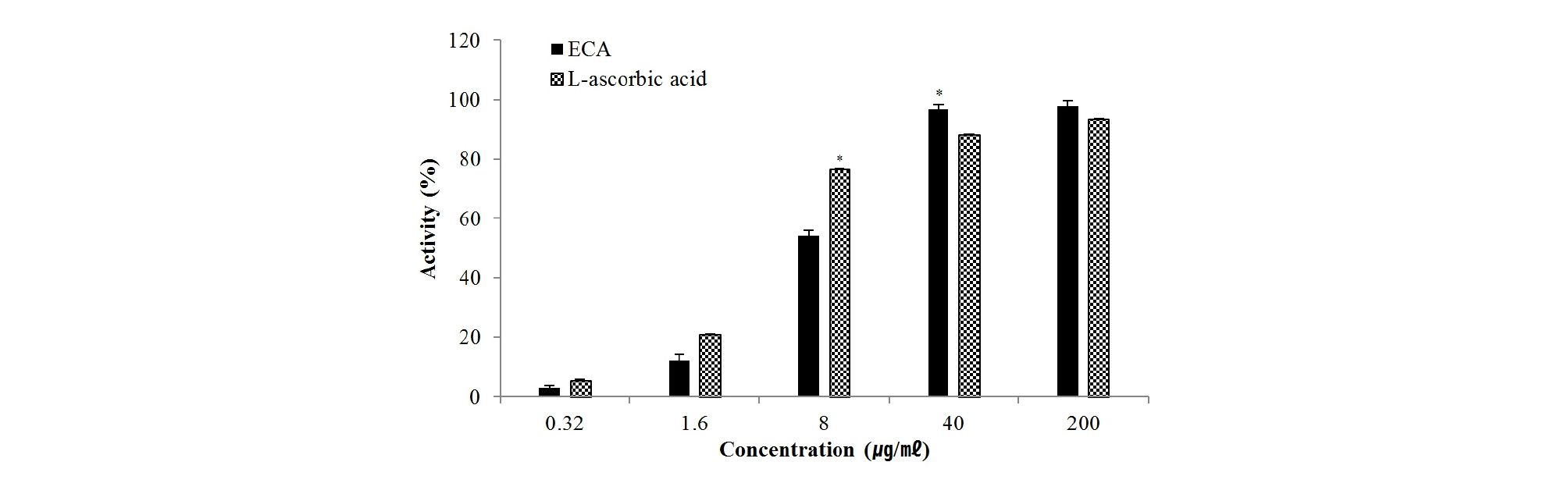
DPPH and ABTS radicals are the most widely used and stable chromogen compounds for measuring the antioxidant activity of biological material. Further, the DPPH radical scavenging and ABTS radical cation decolorization assay can be used to evaluate antioxidant activities in a relatively short time. DPPH is a free radical that accepts an electron or hydrogen for conversion into a stable molecule. Upon interacting with DPPH, antioxidants transfer an electron or hydrogen atom to DPPH and thus neutralize its free radical character (Luis et al., 2009; Sieniwska et al., 2010). As shown in Fig. 2, ECA scavenged DPPH radicals in a dose-dependent manner. The IC50 value was 12.4 ㎍/㎖, indicating very high antioxidant activity compared to the IC50 value (5.1 ㎍/㎖) of L-ascorbic acid under the same conditions. Oxidation of ABTS with potassium persulfate to generate ABTS•+ radical cations requires the transfer of one electron. Then, under prolonged oxidative conditions, this radical can be transformed into a dication ABTS2+ (Branchi et al., 2005; Venkatasubramanian and Maruthamuthu, 1989). ECA and L-ascorbic acid scavenged ABTS radical in a dose-dependent manner (Fig. 3). The IC50 values of ECA and L-ascorbic acid were 6.8 ㎍/㎖and 10.5 ㎍/㎖, respectively. DPPH and ABTS radical are the same radical, but DPPH is a free radical while ABTS is a cation radical. Each radical and antioxidant shows a different response (Meir et al., 1995). Therefore, ECA more effectively removed cation radicals than L-ascorbic acid.
Inhibitory effect of ECA against DNA cleavage by oxidative stress
DNA damage is any irreversible covalent modification of a DNA molecule. Oxidative DNA damage represents one of the most frequent results of exposure to external environmental or endogenous genotoxic agents. These reactions are related to the action of ROS such as hydroxyl radicals, superoxide, peroxide, or single oxygen (Cowan, 2001; Vacek et al., 2007). Oxidative DNA damage caused by the Fenton reaction plays a major role in conditions including aging, neurodegenerative disorders (Alzheimer’s disease), cancer, multiple sclerosis, and many others (Demple and Harrison, 1994; Hasty and Vijg, 2002; Kanvah and Schuster, 2004; Poulsen et al., 1998). According to Jang et al. (2016), they reported the relevance between antioxidant activities were related between antioxidant activities and inhibitory effect against DNA cleavage by oxidative stress.
The plasmid DNA cleavage assay using φX-174 RF I plasmid DNA was used to determine whether ECA with antioxidant activity inhibits the oxidative DNA damage induced by hydroxyl radical or Fe2+ ion. Conversion of the supercoiled form of plasmid DNA to open-circular forms has been used as an index of DNA damage (Jung and Surh, 2001). The remaining supercoiled form of the untreated control (line 1) was set to 100% and the Fenton reaction-induced group (line 2) was set to 0% because of oxidative damage. ECA showed inhibitory effects against oxidative DNA damage by Fe2+ ion inducing DNA cleavage (Fig. 4A). ECA inhibited oxidative DNA damage induced by the Fenton reaction in a concentration-dependent manner. The inhibitory effect of ECA at 200 ㎍/㎖was very high (82.8 ± 2.1%). Fe2+ ion mediates the generation of reactive oxygen radicals and leads to DNA damage caused by O2 and H2O2 in the Fenton reaction (Cai et al., 1995). Additionally, ECA showed inhibitory effects against oxidative DNA damage mediated by a hydroxyl radical inducing DNA cleavage (Fig. 4B) in a concentration-dependent manner. The inhibitory effect of ECA at 200 ㎍/㎖was very high (88.0 ± 4.2%). Hydroxyl radicals attack DNA structures and lead to sugar fragmentation, strand scission, and base adducts (Hutchinson, 1985). The plasmid DNA was mainly in the supercoiled form in the absence of hydroxyl radical and Fe2+. During the reaction of hydroxyl radical and Fe2+, the supercoiled form of plasmid DNA decreased and was converted into an open-circular form. ECA significantly reduced oxidative DNA damage in a dose-dependent manner. Therefore, ECA prevented genetic damage possibly through its protective effects against oxidative stress.
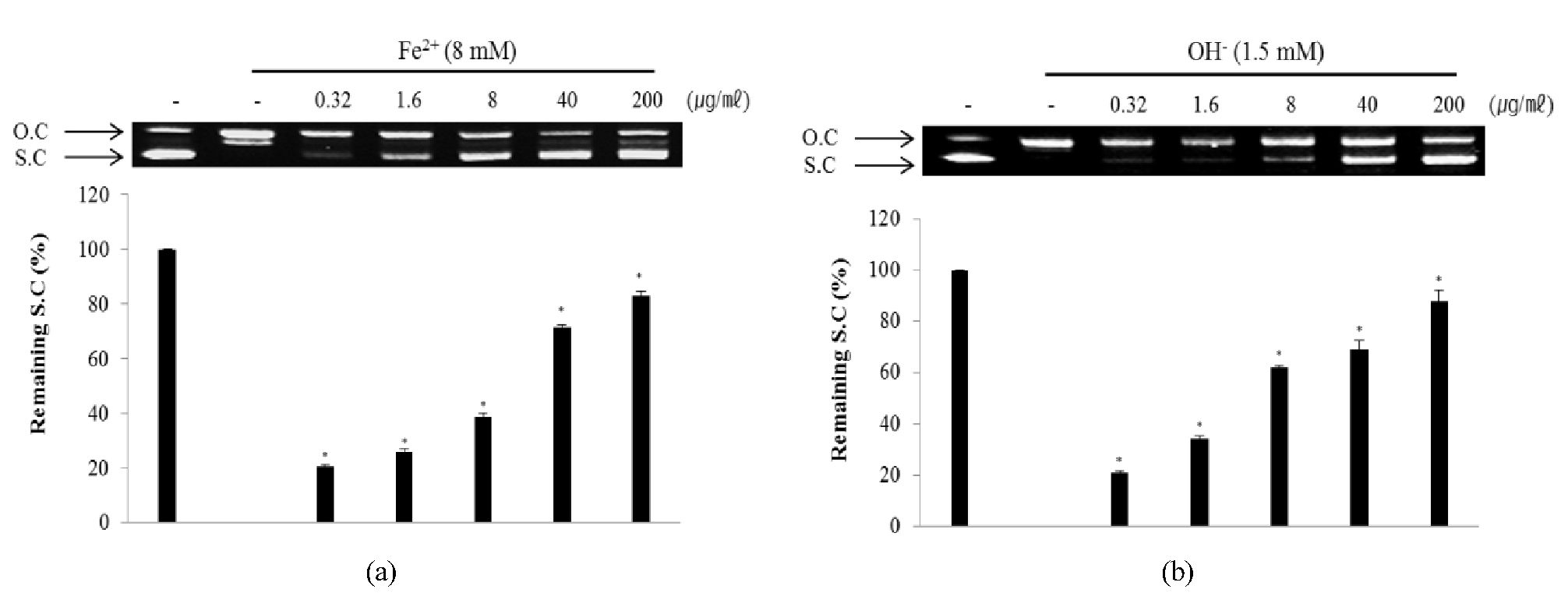
Fig. 4.
Inhibitory effect of ECA against DNA cleavage induced by Fe2+ ion (A) and hydroxyl radical (B). Remaining super-coiled DNA form was quantified using Un-SCAN-IT gel Version 5.1 software (Silk Scientific, Inc., Orem, UT, USA). O.C: Open circular, S.C: Super-coiled. *p < 0.05 as compared to the non-treated control group.
Effect of ECA on DNA damage in NIH/3T3 cells induced by oxidative damage
Cells responding to oxidative DNA damage cause cell-cycle arrest, induce DNA repair, or eliminate the injured cell. Proper cellular responses to DNA double-strand breaks are important for tumor suppression and maintaining genetic stability (Bennett et al., 1993; Mills et al., 2003). One of the first cellular responses to the introduction of double-strand breaks is phosphorylation of H2AX, a sensitive marker for breaks in double-stranded DNA. After DNA double-strand break damage, histone H2AX is phosphorylated at the C-terminal Ser residues (Ser136 and Ser139) (Rogakou et al., 1998). Phosphorylated H2AX known as γ-H2AX can be detected after the introduction of DNA double-strand breaks at sites of DNA damage (Celeste et al., 2003; Sedelnikova et al., 2003). Therefore, γ-H2AX may have distinct roles in DNA damage responses. The p53 pathway is a key effector of the DNA damage response and is activated by several stressors that induce DNA lesions (Phillips and McKinnon, 2007). To evaluate the effect of ECA on DNA damage, the degree of expression of γ-H2AX (Fig. 5A) and p53 (Fig. 5B) protein in NIH/3T3 cells induced by oxidative stress was confirmed. The levels of γ-H2AX and p53 protein in NIH/3T3 cells induced by oxidative DNA damage were down-regulated in a dose-dependent manner by treatment with ECA. Additionally, the mRNA levels of H2AX (Fig. 6A) and p53 (Fig. 6B) were determined to evaluate the inhibitory effect of ECA on oxidative DNA damage. The mRNA levels of γ-H2AX and p53 in NIH/3T3 cells induced by oxidative DNA damage were down-regulated in a dose-dependent manner by treatment with ECA. Abnormalities in the p53 gene are observed in a wide spectrum of diseases and p53 may be related to the cellular response to DNA damage. Based on these results, ECA showed high antioxidant activity and significantly protected DNA from oxidative damage at the cellular level. Therefore, ECA may be useful for developing chemopreventive or therapeutic agents for diseases caused by oxidative DNA damage, such as cancer or inflammation.
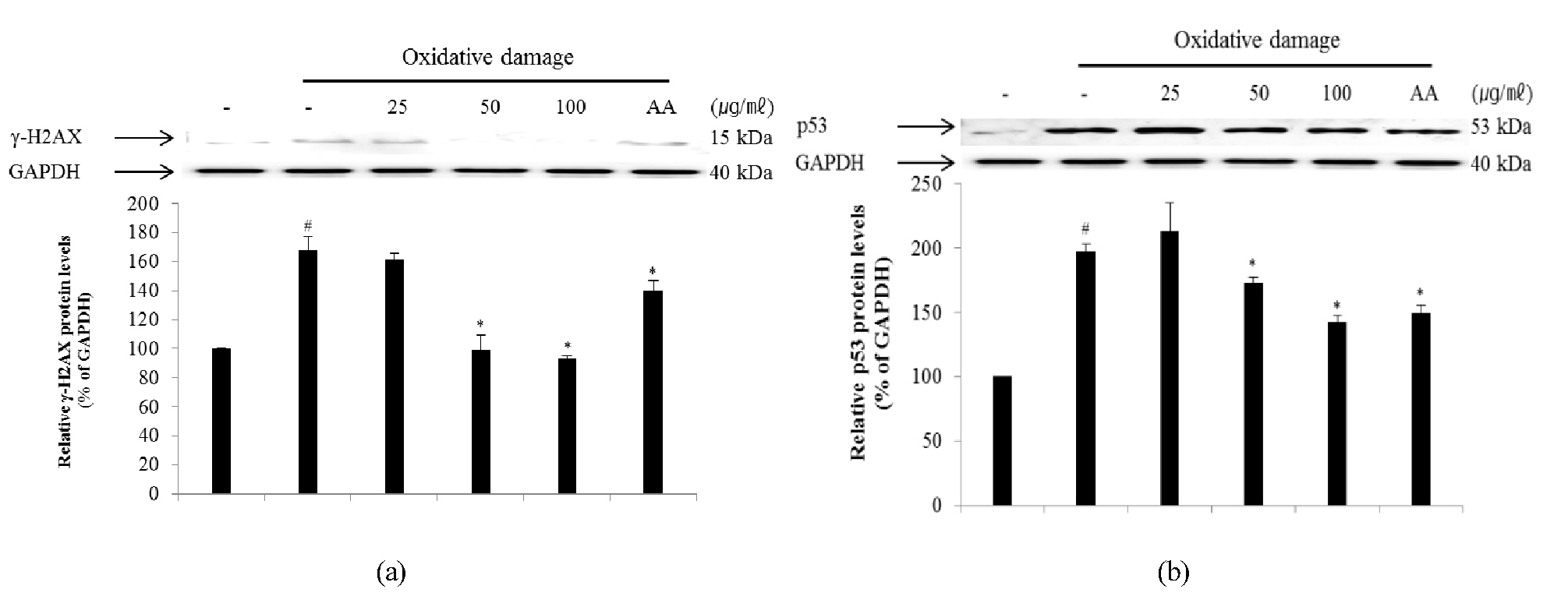
Fig. 5.
Inhibitory effect of ECA on the expression of γ-H2AX (A) and p53 (B) proteins in NIH/3T3 cells induced by oxidative damage. Relative ratio was quantified after normalization to GAPDH. Each value was then determined relative to the non-treated (NT) control group, which was set to 100%. Results are expressed as the mean ± SD for each group from three independent experiments. #, Significant compared to NT, *p < 0.05; *, p < 0.05 versus ECA-untreated oxidative stress-treated group. Each band was quantified using Un-SCAN-IT gel Version 5.1 software (Silk Scientific, Inc.).
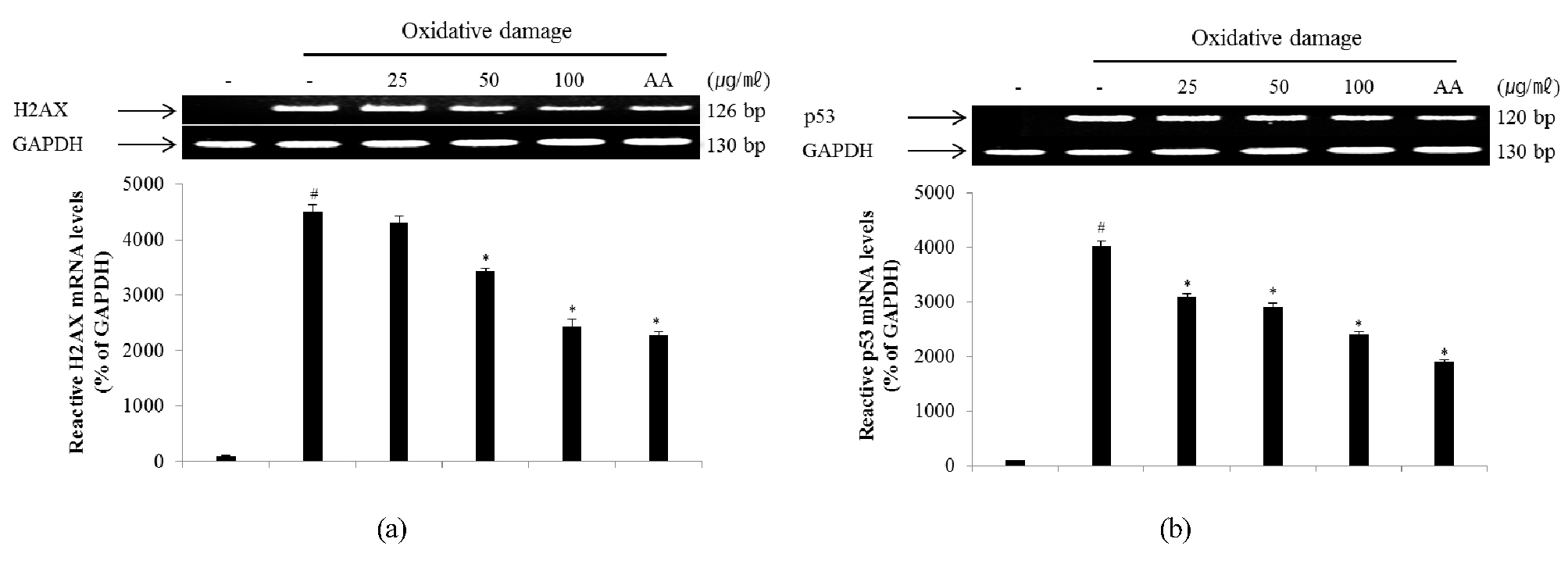
Fig. 6.
Inhibitory effect of ECA on the expression of γ-H2AX (A) and p53 (B) mRNA in NIH/3T3 cells induced by oxidative damage. Relative ratio was quantified after normalization to GAPDH. Each value was then expressed relative to the non-treated (NT) control group, which was set to 100%. Results are expressed as the mean ± SD for each group from three independent experiments. #, Significant compared with NT, *p < 0.05; *, p < 0.05 versus ECA-untreated oxidative stress-treated group. Each band was quantified using Un-SCAN-IT gel Version 5.1 software (Silk Scientific, Inc.).