Introduction
Materials and Methods
Chemicals
Preparation of S. rebaudiana flower extract
Determination of total phenolic and total flavonoid content
Determination of DPPH radical scavenging activity
Determination of hydrogen peroxide scavenging activity
Determination of the reducing power of Stevia-F
Determination of NO scavenging activity
Effect of Stevia-F on α-glucosidase activity in cell-free system
Effect of Stevia-F on tyrosinase inhibitory activity in cell- free system
Cell culture
Cell viability
Effect of Stevia-F on the production of melanin in B16F10 cells
Effect of Stevia-F on cellular tyrosinase activity
Statistical analysis
Results
Total content of phenolics and flavonoids in Stevia-F
Antioxidant capacity of Stevia-F
Effect of Stevia-F on the tyrosinase and α-glucosidase activities
Effect of Stevia-F on the viability of B16F10 cells
Inhibitory effect of Stevia-F on melanin production in B16F10 cells
Effect of Stevia-F on tyrosinase activity stimulated by α- MSH
Discussion
Introduction
Skin is a very complex body organ composed of three layers, viz. the epidermis, dermis, and hypodermis. It plays critical roles in protecting the body from external stimuli, infection, and loss of moisture. Melanin, produced by melanocytes located in the stratum basale, is one of the important factors determining the color of skin (Cichorek et al., 2013). Unlike in the Western world, light complexion of the skin is considered to be an attribute of youthfulness and beauty in eastern societies. Presence of high levels of reactive oxygen species (ROS) stimulates keratinocytes to increase the release of α-MSH, a melanin stimulating hormone, and activates molecules, such as cytokines and tyrosinase, to induce abnormal melanogenesis (Costin and Hearing, 2007). In addition to the suppression of oxidative stress in the skin, one of the most characterized cellular targets of skin-whitening agents are inhibitors of tyrosinase, which is the key enzyme in melanogenesis (Gillbro and Olsson, 2011). Skin-whitening products are used to acquire a lighter skin tone and are also used in the clinical treatment of photoaging and melanin pigmentary disorders, such as melasma and post-inflammatory hyperpigmentation (Bin et al., 2016). Developing and customizing preparations for bleaching hyperpigmentation lesions or achieving overall skin whitening is one of the challenges in the cosmetics industry, especially in East Asia.
Stevia (Stevia rebaudiana Bertoni) is a perennial plant belonging to the Asteraceae family (Lemus-Mondaca et al., 2012). The leaves of Stevia contain high amounts of steviol glycoside, which is mainly used as a calorie-free sweetener (Samuel et al., 2018). Besides steviol glycosides, S. rebaudiana possesses more than 100 phytochemicals and other compounds with antioxidant and medicinal properties (Lemus-Mondaca et al., 2012). For agricultural and industrial applications, leaf is the most widely used part of S. rebaudiana, and its usage has been reviewed elsewhere (Lemus-Mondaca et al., 2012). Because of poor seed germination, Stevia is propagated through stem cuttings (Lemus-Mondaca et al., 2012), and therefore, its flowers are not necessarily required for propagation.
In recent years, many studies have focused on the development of cosmetic ingredients that can provide safe and natural ways of getting rid of skin coloration. In this context, we investigated the antimelanogenic activities and antioxidant properties of Stevia flower extract (Stevia-F). For this, we determined the total flavonoid (TFC) and phenolic (TPC) contents of Stevia-F. The antioxidative potential of Stevia-F was determined based on the reducing power and scavenging of 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH), hydrogen peroxide, and nitric oxide (NO). Moreover, the antimelanogenic effect of Stevia-F on B16F10 cells was investigated through its inhibition of melanin production, and activities of tyrosinase and α-glucosidase, which is an essential modulator of tyrosinase activity.
Materials and Methods
Chemicals
Dimethylsulfoxide (DMSO), DPPH, β-phycoerythrin, mushroom tyrosinase, α-melanocyte stimulating hormone (α- MSH), L-3,4-dihydroxyphenylalanine (L-DOPA), methanol, phenylmethylsulfonyl fluoride (PMSF), and deionized distilled water were purchased from Sigma-Aldrich (St. Louis, MO, USA). Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were obtained from Gibco (Grand Island, NY, USA). Unless indicated otherwise, all other chemicals were purchased from Sigma-Aldrich.
Preparation of S. rebaudiana flower extract
The plants were collected from the Jecheon area during the period from August to October 2017, and were identified by Dr. H. J. Koo of Korea National College of Agriculture and Fisheries. Voucher specimens were deposited at the herbarium of the Department of Medicinal and Industrial Crops, Korea National College of Agriculture and Fisheries (Jeonju, Korea). The flowers of S.rebaudiana were dried in an oven at 60℃ for 24 h to remove the moisture. The air-dried flowers (3 g) were powdered and then extracted with 30 mL water. The extract was subsequently filtered to remove any particulate matter present, and was evaporated under reduced pressure to a viscous dark mass with a percentage yield of 4.5% (w/w). This was then lyophilized to obtain a powder, which was stored at –20℃ for further experiments.
Determination of total phenolic and total flavonoid content
Total phenolic content was determined using the Folin- Ciocalteu method (Sasidharan et al., 2010). The extract was mixed with Folin–Ciocalteu’s phenol reagent (50 g/100 mL) and then sodium carbonate (2 g/100 mL) was added to the mixture, and the final volume was made up to 5 mL with deionized water. The mixture was allowed to stand at 25℃ for 30 min and the absorbance was measured at 750 ㎚ using a UV/VIS spectrophotometer (Shimadzu, Kyoto, Japan). The total phenolic content of Stevia-F was calculated using a standard curve prepared using gallic acid and was expressed as ㎎ of gallic acid equivalent (GAE)/100 g.
Total flavonoid content was determined using the aluminum chloride colorimetric method (Chang et al., 2002; Marinova et al., 2005 ). The extract was mixed with aluminum chloride hexahydrate (10 g/100 mL) and then 95% ethanol (EtOH) and 1 M potassium acetate were added, and the final volume was made up to 5 mL with deionized water. After mixing, the solution was incubated for 40 min at 25℃. The absorbance of the reaction mixture was measured at 415 ㎚ using a UV/VIS spectrophotometer. The total flavonoid content in the sample was calculated from the standard curve and expressed as ㎎ of quercetin equivalent (QE)/100 g. All the experiments were carried out, at least, in triplicate.
Determination of DPPH radical scavenging activity
Different concentrations of Stevia-F were adjusted to 100 µL with reaction mixture and then reacted with l00 µL of 0.4 mM DPPH solution in 99% EtOH. After vigorous shaking, reaction mixtures were allowed to reach a steady state at 25℃ for 30 min. Decolorization of DPPH was evaluated by measuring the absorbance at 540 ㎚ using a microplate reader VICTOR X3 (PerkinElmer, Waltham, MA, USA). The concentration required to inhibit 50% of DPPH radical formation (IC50) was calculated from the graph by plotting the inhibition percentage against the tested concentrations of Stevia-F.
Determination of hydrogen peroxide scavenging activity
Different concentrations of Stevia-F were adjusted to 20 µL with reaction mixture and then reacted with 20 µL of 1.0 M H2O2 and 0.1 M phosphate buffer at 37℃ for 5 min. After mixing, 30 µL of 1.25 mM ABTS (2,2′-Azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) and 30 µL of 1 unit/mL peroxidase were added, and after incubating for 10 min at 37℃, absorbance was measured at 405 ㎚ using a UV/VIS spectrophotometer. The IC50 for inhibition of hydrogen peroxide formation was calculated from the graph by plotting inhibition percentage against the tested Stevia-F concentrations.
Determination of the reducing power of Stevia-F
Different concentrations of Stevia-F were incubated with potassium ferricyanide (1 g/100 mL) and 0.2 M sodium phosphate buffer (pH 6.6) at 50℃ for 20 min. The reaction was terminated by addition of TCA solution (10 g/100 mL); after centrifugation at 3000 × g for 10 min, the supernatant was mixed with ferric chloride (0.1 g/100 mL) and reaction was conducted at 25℃ for 10 min. The absorbance was measured at 700 ㎚ using a UV/VIS spectrophotometer. The concentration of extract resulting in an absorbance of 0.5 (IC50) was calculated using a calibration graph made by plotting the concentration against the absorbance at 700 ㎚.
Determination of NO scavenging activity
Different concentrations of Stevia-F were incubated with 10 mM sodium nitroferricyanide (III) dihydrate at 25℃ for 150 min. The reaction mixture was then treated with 1% sulfanilamide (dissolved in 30% acetic acid) for 5 min and further incubated with 0.1% N-(naphthyl)ethylenediamine dihydrochloride (dissolved in 60% acetic acid) at 25℃ for 30 min. The absorbance was measured at 520 ㎚ using a microplate reader VICTOR X3.
Effect of Stevia-F on α-glucosidase activity in cell-free system
The inhibition of α-glucosidase activity was evaluated using a published method (Kim, J.S. et al., 2000), with some modifications. Briefly, α-glucosidase (Saccharomyces cerevisiae, Sigma-Aldrich, USA) was dissolved in 100 mM of sodium phosphate buffer (pH 6.8) containing 200 ㎎ of bovine serum albumin (Merck, Germany). The reaction mixture consisting of 10 µL sample was premixed with 90 µL α- glucosidase (1 unit/mL). After preincubation at 37℃ for 15 min, 100 µL of 1 mM p-nitrophenyl α-D-glucopyranoside (Sigma-Aldrich, Switzerland) was added and the mixture was incubated at 37℃ for 5 min. α-glucosidase activity was determined spectrophotometrically at 405 ㎚ using a microplate reader VICTOR X3.
Effect of Stevia-F on tyrosinase inhibitory activity in cell- free system
The inhibition of tyrosinase activity was determined by a colorimetric method (Lee et al., 2017). Briefly, 40 µL of mushroom tyrosinase (110 units/mL) was added to 100 µL of reaction mixture containing 175 mM sodium phosphate buffer (pH 6.8) and 40 µL of 10 mM L-DOPA was added in the presence or absence of the sample. The reaction was conducted at 25℃ for 5 min and the absorbance was measured at 490 ㎚ using a microplate reader VICTOR X3. Tyrosinase inhibitory activity (%) was calculated using the following equation: % inhibition = [1-(Sample with enzyme - Sample without enzyme)/(Blank with enzyme - Sample without enzyme)] × 100.
Cell culture
B16F10 mouse melanoma cells were obtained from Korean Cell Line Bank (Seoul, Korea) and maintained in DMEM containing 10% FBS and 1% penicillin in a humidified atmosphere of 5% CO2 at 37℃.
Cell viability
Cell viability was measured using the quantitative colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, as previously reported (Koo et al., 2017). Exponentially growing B16F10 cells were seeded at 1 × 104 cells/well in 96-well tissue culture plates and treated with different doses (1, 10, 50, 100 ㎍/mL) of Stevia-F for 24 and 48 h. After incubation with MTT (150 ㎍/mL) for 4 h, the formazan crystals formed were dissolved in DMSO, and the optical density was measured at 540 ㎚ using a microplate reader VICTOR X3. Cytotoxicity was expressed as a percent of untreated control cells.
Effect of Stevia-F on the production of melanin in B16F10 cells
B16F10 cells were treated with α-MSH (100 nM) in the presence or absence of different doses of Stevia-F (1–100 ㎍/ mL) for 48 h. The cells were washed with D-PBS and lysed with lysis buffer containing 50 mM sodium phosphate buffer (pH 6.8), 1% Triton X-100, and 0.1 mM PMSF. After collecting the supernatant by centrifugation, the pellets were dissolved in 1 N NaOH for 1 h at 60℃ and the absorbance was measured at 490 ㎚ using a microplate reader VICTOR X3.
Effect of Stevia-F on cellular tyrosinase activity
B16F10 cells were treated with α-MSH (100 nM) in the presence or absence of different doses of Stevia-F (1–100 ㎍/mL) for 48 h. The culture medium was then removed, and the cells were washed with D-PBS and lysed with lysis buffer containing 50 mM sodium phosphate (pH 6.8) buffer, 1% Triton X-100, and 0.1 mM PMSF. After centrifugation, tyrosinase activity was determined in the cell supernatant by addition of the reaction mixture (40 µL of 100 mM sodium phosphate buffer (pH 6.8) and 160 µL of 10 mM L-DOPA) in the presence of cell lysate (40 ㎍) for 1 h. The absorbance was measured at 490 ㎚ using a microplate reader VICTOR X3.
Statistical analysis
Each experiment was repeated three or four times, and the results of a representative experiment are shown. The results are expressed as means ± SEM and were analyzed using one-Way ANOVA followed by Turkey’s test (Systat Software Inc., San Jose, CA, USA). A statistical probability of p< 0.05 was considered significant.
Results
Total content of phenolics and flavonoids in Stevia-F
Plant phenolic compounds are among the most important antioxidants in fruits, seeds, and vegetables (Cai et al., 2004). The total flavonoid content in Stevia-F was determined to be 8.64 ± 0.23 ㎎ QE/100 g and total phenolic content was 631.5 ± 2.01 ㎎ GAE/100 g (Table 1).
Table 1. Total phenolic and total flavonoid content of Stevia rebaudiana flower extract
| Total phenolic (㎎GAEz/100 g) | Total flavonoid (㎎QEy/100 g) | |
| Water extract of dried flowers of S. rebaudiana (Stevia-F) | 631.5 ± 2.01x | 8.64 ± 0.23x |
Antioxidant capacity of Stevia-F
The antioxidant activities of Stevia-F, at doses up to 100 ㎍/mL, were evaluated in terms of the reducing power (Fig. 1A), and scavenging potential of DPPH radical (Fig. 1B), hydrogen peroxide (Fig. 1C), and nitric oxide (Fig. 1D). The IC50, determined using the regression equation, of Stevia-F for reducing power was 5541 ㎍/mL and the values for the DPPH radical, hydrogen peroxide, and NO scavenging activities were 131.38, 464.34, and 10.44 ㎍/mL, respectively. Although Stevia-F showed less antioxidant capacity than ascorbic acid (Fig. 1), NO scavenging activity of Stevia-F was higher than that of ascorbic acid at all concentrations.
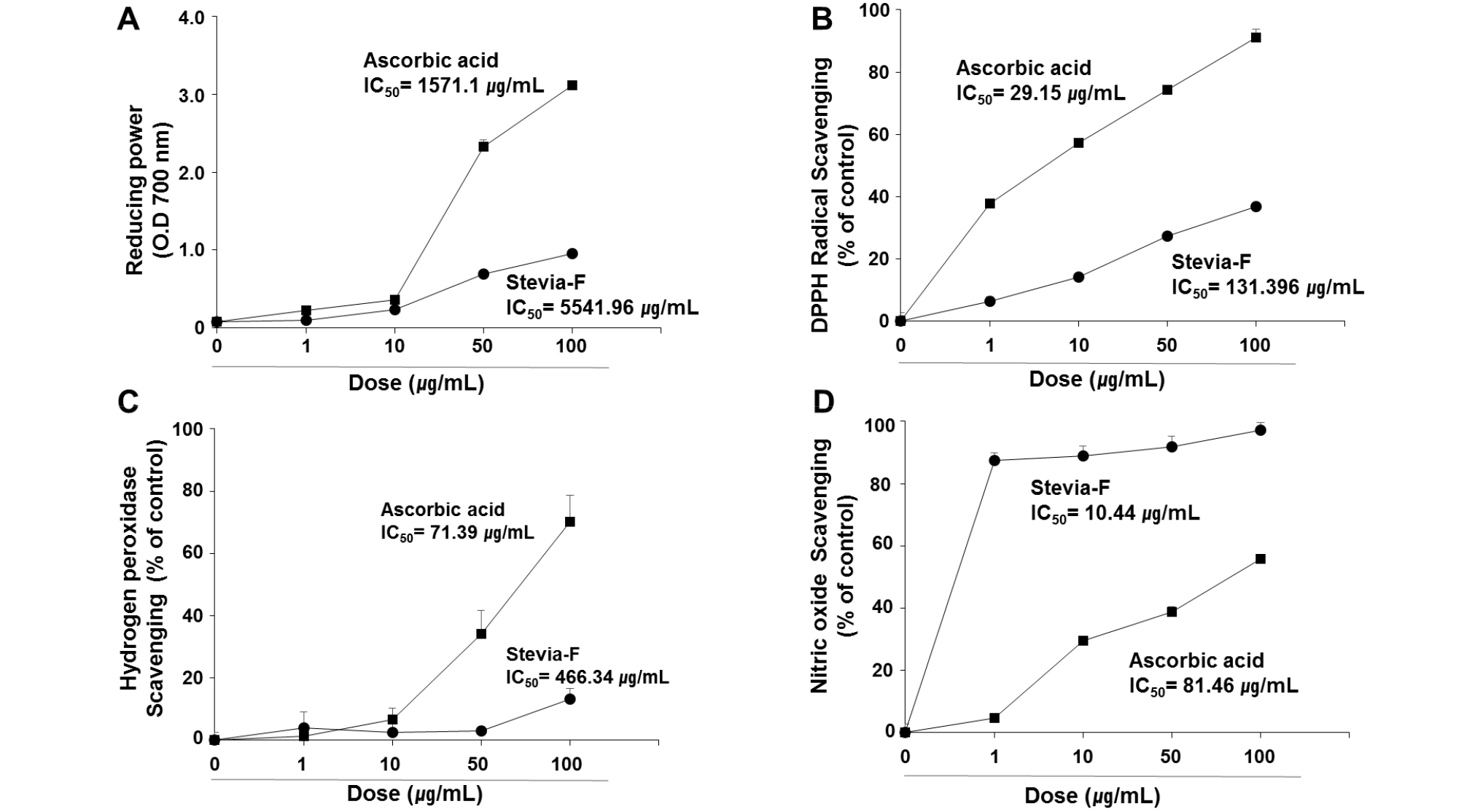
Fig. 1.
Antioxidant activities of Stevia-F. Antioxidant activities of Stevia-F were determined in terms of reducing power (A), and 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) (B), hydrogen peroxide (C), and nitric oxide (NO) (D) scavenging activities. The effective concentration required for 50% scavenging activity (IC50) was determined using the regression equation. Data are means ± SEM (n = 4). Stevia-F; aqueous extract of Stevia rebaudiana flower.
Effect of Stevia-F on the tyrosinase and α-glucosidase activities
Suppression of melanin production is one of the key events in the skin whitening process. Polyphenols belong to the largest group of tyrosinase inhibitors and are potential sources of skin-whitening agents (Chang, 2009). The α-glucosidase present in endoplasmic reticulum is involved in the N-glycan processing of tyrosinase and, therefore, inhibition of this enzyme results in the suppression of melanin production due to the failure of the transport of tyrosinase to melanosomes (Petrescu et al., 1997). Our results show that the contents of phenolics and flavonoids as well as the antioxidant activities of Stevia-F were high (Fig. 1). To determine the antimelanogenic effect of Stevia-F, we determined its inhibitory effect on tyrosinase and α-glucosidase activities in a cell-free system. As shown in Fig. 2, the IC50 value of Stevia-F on tyrosinase and α-glucosidase activities were 134.74 and 114.81 ㎍/mL, respectively.
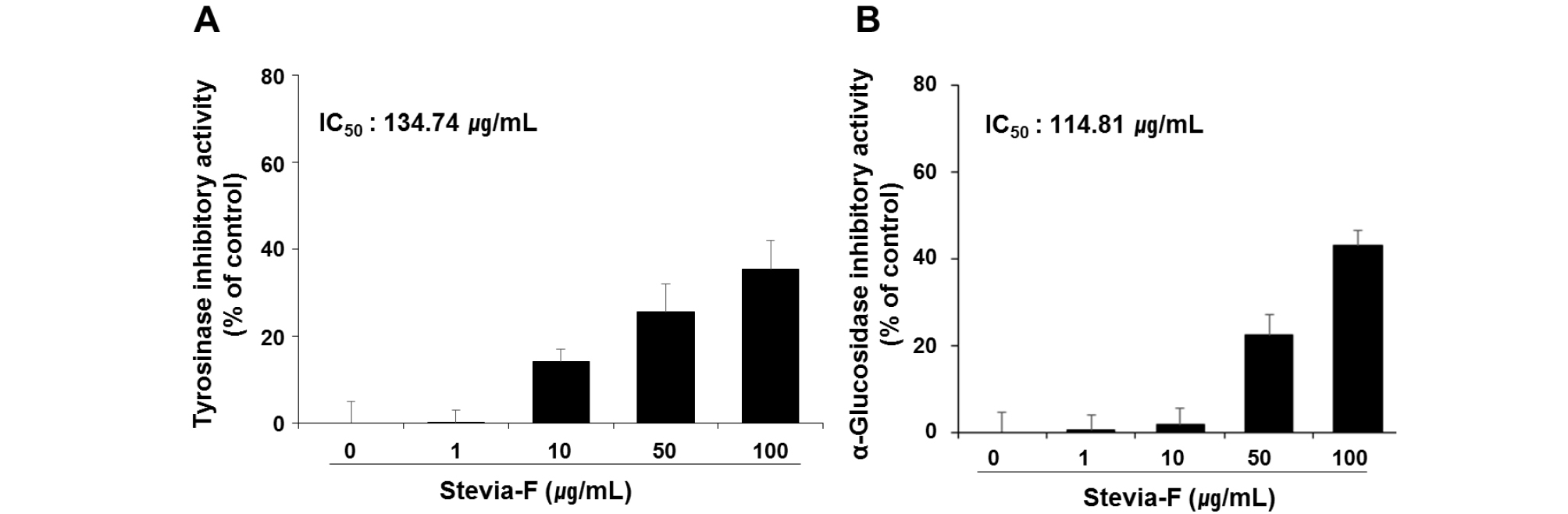
Fig. 2.
Inhibitory effects of Stevia-F on tyrosinase and α-glucosidase activities in cell-free system. Tyrosinase and α-glucosidase regulatory activities were determined using a colorimetric method. The effective concentration required for 50% scavenging activity (IC50) was determined using the regression equation. Data are means ± SEM and are expressed as percentages of untreated control. Stevia-F; aqueous extract of Stevia rebaudiana flower.
Effect of Stevia-F on the viability of B16F10 cells
MTT assay was conducted at 24 and 48 h to determine the cytotoxicity of Stevia-F in B16F10 cells. No significant cytotoxicity was observed at any of the concentrations up to 100 ㎍/mL (Fig. 3).
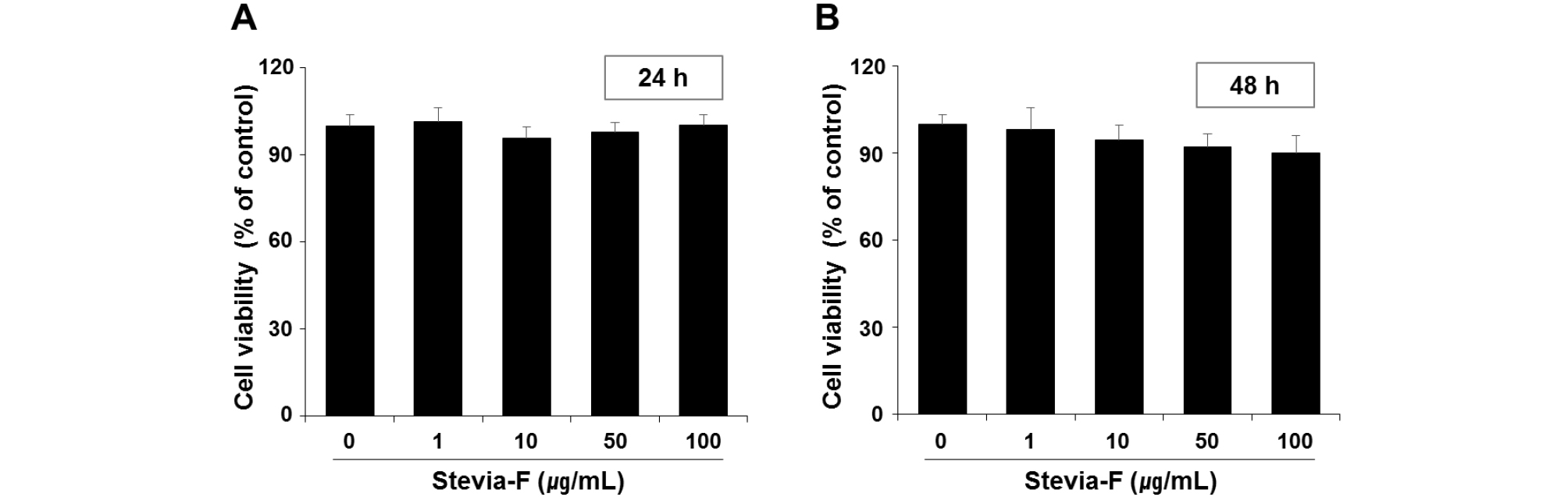
Fig. 3.
Cytotoxicity of Stevia-F on B16F10 melanocytes. Cell viabilities were measured using MTT assay at 24 (A) and 48 h (B) after treatment with different concentrations (1–100 ㎍/mL) of Stevia-F. Data are means ± SEM (n = 5) and are expressed as percentages of untreated control. Stevia-F; aqueous extract of Stevia rebaudiana flower.
Inhibitory effect of Stevia-F on melanin production in B16F10 cells
The effect of Stevia-F on the melanin production in B16F10 cells was determined in the presence α-MSH (100 nM). Arbutin was used as a positive control for determination of melanin production because it inhibits tyrosinase and, thus, prevents the formation of melanin. Following α-MSH stimulation, the melanin production was significantly increased (160.45 ± 2.21% vs. untreated control, p < 0.05). However, the α-MSH-stimulated increase in melanin production was significantly suppressed by Stevia-F treatment in a dose- dependent manner (Fig. 4A). This suggests that Stevia-F can suppress α-MSH-mediated stimulation of melanin production.
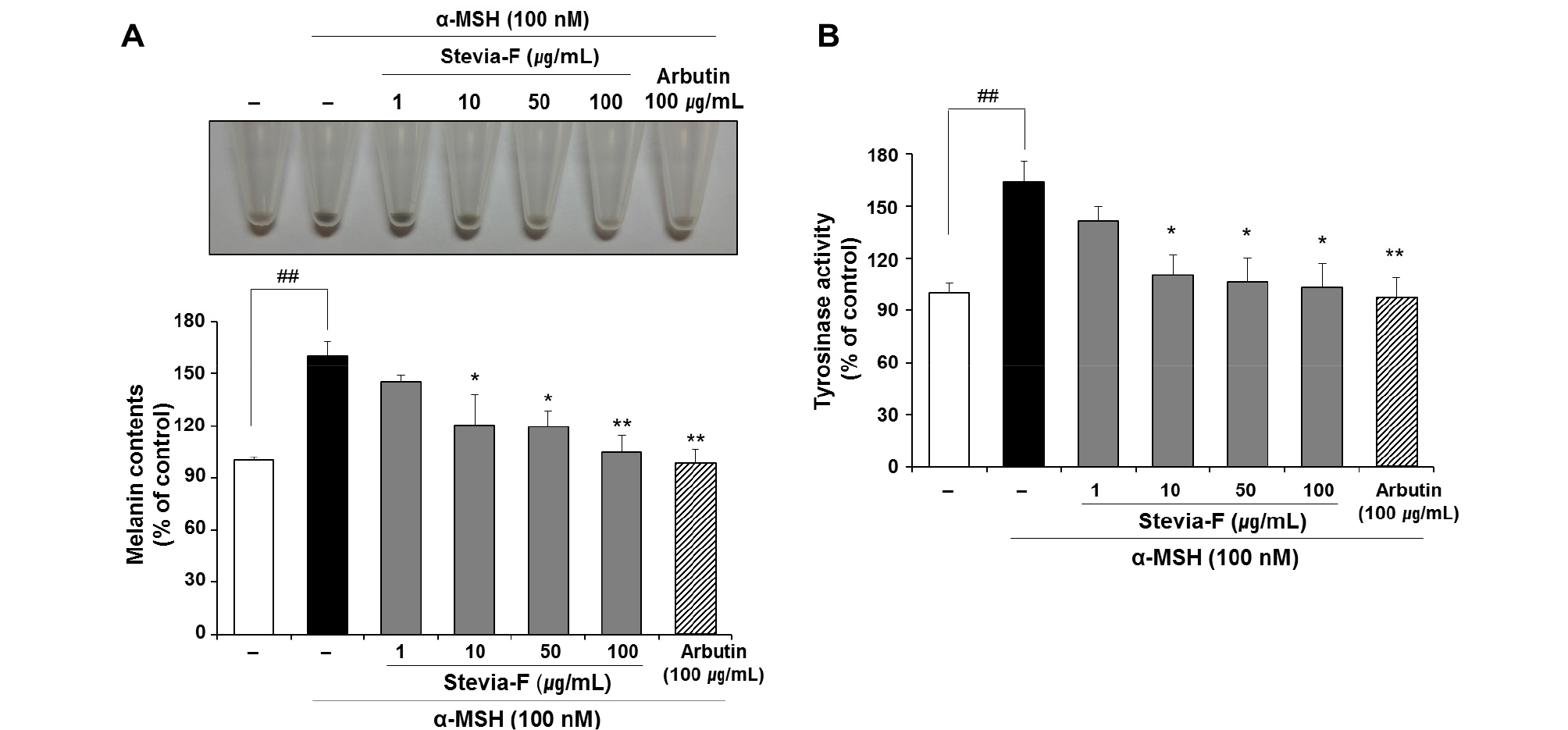
Fig. 4.
Effects of Stevia-F on melanin production and tyrosinase activity in α-MSH-stimulated B16F10 cells. Cells were exposed to α-melanocyte stimulating hormone (α-MSH, 100 nM) in the presence or absence of Stevia F (1–100 ㎍/mL) for 48 h. Images of the harvested B16F10 cells are presented (A, upper panel) and melanin production rate is expressed as a percentage of the activity in the untreated control cells (A, lower panel). Tyrosinase activity was determined by a colorimetric method and is expressed as a percentage of the activity in the untreated control cells (B). Data are means ± SEM (n = 5). ##p < 0.01 vs. untreated control and *p < 0.05, **p < 0.01 vs. α-MSH-treated control. Stevia-F; aqueous extract of S. rebaudiana flower.
Effect of Stevia-F on tyrosinase activity stimulated by α- MSH
Determination of the change in tyrosinase activity in cells treated with a herb extract is an important method for confirming the whitening effect on cells. Stevia-F suppressed the melanin production in α-MSH-induced B16F10 cells (Fig. 4A). In addition, in in vitro cell-free system, Stevia-F showed a direct inhibitory effect on tyrosinase activity (Fig. 2A). We further tested the inhibition of tyrosinase activity by Stevia-F (10 to 100 ㎍/mL, p < 0.05) in α-MSH-stimulated B16F10 cells. Consistent with the results of changes in the melanin content (Fig. 4A), Stevia-F inhibited the tyrosinase activity in B16F10 cells (Fig. 4B).
Discussion
In this study, we investigated the antioxidant potential of Stevia-F in terms of its phenolic and flavonoid content (Table 1), and ROS scavenging activities (Fig. 1). Stevia-F caused inhibition of tyrosinase and α-glucosidase activities (Fig. 2). The treatment also resulted in the inhibition of α-MSH- stimulated melanin production, which might, in part, be associated with the inhibition of tyrosinase by Stevia-F.
The cultivation of Stevia has spread from its native habitat in the Amambay region of north east Paraguay to other regions of the world. Being a natural source of low-calorie sweeteners, Stevia has attracted attention as a sugar substitute for diabetic patients, children, and people interested in lowering their calorie intake (Lemus-Mondaca et al., 2012). In addition, functional and health-promoting properties of Stevia have been under investigation for use in obesity, diabetes mellitus (Singla et al., 2019), heart disease (Ragone et al., 2017), and dental caries (Ghanta et al., 2007). Most of the studies on antimicrobial and antioxidant activities of Stevia have used leaves (Lemus-Mondaca et al., 2012); the use of flower parts has been rare and there are few reports on their use for antimelanogenic purpose. Thus, we evaluated the antimelanogenic potential of Stevia-F. Antioxidants have been recognized as important functional ingredients of cosmetic materials, which inhibit aging and pigmentation of skin by scavenging ROS. Stevia-F has a high phenolic and flavonoid content and exhibits radical scavenging activities (Fig. 1). Determination of the change in tyrosinase activity in cells treated with the extracts is a useful method for confirming the depigmentation activity of herbs. Our results suggest that Stevia-F has a significant potential for inhibiting the tyrosinase activity in both cellular (Fig. 4B) and cell-free systems (Fig. 2B). In addition, α-glucosidase, which is involved in the transport of tyrosinase to melanosomes, could be inhibited by treatment with Stevia-F (Fig. 2B). These inhibitory effects of Stevia-F resulted in the suppression of α- MSH-stimulated increase in melanin production in B16F10 cells (Fig. 4A). Ultraviolet (UV) radiation is an important environmental stimulus that effects human skin pigmentation by inducing the secretion of NO from keratinocytes; melanin production is enhanced by inducible NO synthase and secreted NO (Roméro-Graillet, C. et al., 1997). NO scavengers inhibit the UV radiation-mediated increase in melanin production. Stevia-F showed a remarkable NO scavenging activity, which was better than that of ascorbic acid (Fig. 1D). This finding also raises the possibility that Stevia-F might suppress UV radiation-induced melanin production. However, this should be investigated further in future studies.
In conclusion, our results demonstrate that Stevia-F has antioxidant and antimelanogenic potential. The use of Stevia flowers for cosmetic purposes would enhance the possibility for their industrial and agricultural applications.




