Introduction
Materials and Methods
Plant materials and in vitro culture
Long-term in vitro culture of cell lines
Shoot induction and adventitious root induction from long-term cultured cultures
Sucrose treatment
Acclimatization of regenerants
Flow Cytometry Analysis
Statistical analysis
Results and Discussion
Effect of plant growth regulators on adventitious buds (shoots) of long-term cultured cell lines
Effect of plant growth regulators on adventitious roots of long-term cultured cell lines
Effect of sucrose concentration on organogenesis of long-term cultured cell lines
Acclimatization of regenerants
Flow cytometry of regenerants and calli derived from long-term cultured cell lines
Introduction
In vitro propagation is an essential requirement in plug seedling production, as well as in expanding of naturally occurring germplasm. The plant regeneration pathway by in vitro culture is divided into two pathways, one involves direct organogenesis, showing adventitious root induction and adventitious bud induction (shooting), and the other involves somatic embryogenesis or non-somatic embryogenesis via didiferentiated cells or calli (Cho and Byeon, 2011; Hans et al., 2014; Kamińska and Sliwinska, 2023; Lee et al., 2023c; Murashige and Skoog, 1962; Rebouillat et al., 2009; Seo, 2018).
Long-term in vitro maintenance is essential for the in vitro culture system and the expansion of tissue culture potentials (Zhang et al., 2000). Long-term culture or long-term maintenance in vitro have been studied in various kinds of plants, barley (Armando et al., 2011), cereals (Nabors et al., 1983), manilagrass (Chai et al., 2011), orchid (Ryu et al., 2013b), rice (Bajaj and Rajam, 1995, 1996; Kavi Kishor and Reddy, 1986; Yang et al., 1999), wheat with long-term retention (Sharma et al., 2008) and white clover (White, 1984). Cytokinins are important in long-term cultures of plant cell lines (Bajaj and Rajam, 1995, 1996; Chai et al., 2011; Yang et al., 1999). This long-term potential is essential in wheat transformation (Zhang et al., 2000). Even though the regeneration has been reported in I. cylindrica ‘Rubra’ (Kang et al., 2021; Lee et al., 2023a, 2023b, 2023c), however, long-term culture in vitro has not been reported in I. cylindrica ‘Rubra’.
I. cylindrica ‘Rubra’ found in North America, Asia, Africa, tropical and subtropical regions, belongs to the Poaceae family. It is a type of bio-energy plant resource (Goh et al., 2011) and is used for architectural purposes.
Researches for in vitro regeneration of Gramineae/Poaceae have been reported using various kind of variety, organ parts and kinds of plant growth regulators (Cho and Byeon, 2011; Umami et al., 2012). As for plant organs, they include seeds with embryos (Cho and Byeon, 2011; Goh et al., 2011), immature ovary and pollen, immature inflorescence and meristem (Umami et al., 2012). Regeneration by in vitro culture in cogongrass has been reported (Shigeki et al., 2009; Umami et al., 2012). In this research, we evaluated the regeneration ability and acclimatization process using regenerants from long-term (5-year) cultured calli and checked the ploidy level of regenerants in I. cylindrica ‘Rubra’.
Materials and Methods
Plant materials and in vitro culture
Callus was induced from the culture of meristem containing tissue of I. cylindrica ‘Rubra’ on MS (Murashige and Skoog, 1962) medium containing 0.1 ㎎/L 2,4-D and 2.0 ㎎/L BA. Calli were proliferated on MS medium supplemented with 0.1 ㎎/L 2,4-D, and adventitious buds (shoots) were induced on MS medium containing 0.1 ㎎/L BA and 0.1 ㎎/L 2,4-D (Kang et al., 2021; Lee et al., 2023a, 2023b, 2023c; Umami et al., 2012). The cultures were cultured at 26±2℃, 14/10h (day/night), 25μmol/㎡/s. The pH of the MS medium was adjusted to pH 5.80±0.05 before autoclaving.
Long-term in vitro culture of cell lines
Using the calli mentioned above, they were sub-cultured on MS medium with 0.1 ㎎/L 2,4-D, and adventitious buds (shoots) were sub-cultured on MS medium with 0.1 ㎎/L BA and 0.1 ㎎/L 2,4-D by 6 to 8-week intervals from June 2018, to June 2023.
Shoot induction and adventitious root induction from long-term cultured cultures
For shoot induction the long-term cultured calli were treated on MS medium with 0.1 ㎎/L BA and 0.01 ㎎/L of auxins (IAA, NAA, IBA and 2,4-D) (Table 1), and were treated on MS medium with 2.0 ㎎/L TDZ and 0.01 ㎎/L of auxins (IAA, NAA, IBA and 2,4-D) (Table 2). For adventitious root induction the long-term cultured calli were treated on MS medium with 0.01 ㎎/L of auxins (NAA, IBA and 2,4-D) (Table 3). Characteristics of organogenesis, such as shoot number, adventitious root number and adventitious bud induction (%) were measured (Table 1 to Table 4) after 12 weeks. Callus induction (%), adventious bud induction (%) were calculated as follows. Regeneration efficiency (%) = number of shoot induced calli cluster/number of calli cluster×100.
Table 1.
Characteristics of in vitro growth by 12 weeks BA and auxin treatments on MS medium in cell lines of Imperata cylindrica ‘Rubra’ cultured for 5-year
|
PGRsz treatment (㎎/L) |
REy (%) |
Shoot no./ Calli cluster |
Shoot length (㎝) |
Fresh weight (g) |
Adventitious root number |
Adventitious root length (㎝)x |
| BA 0.1 + IAA 0.01 | 100 | 19.33±1.15c | 5.60±0.52a | 0.533±0.05a | 30.33±0.57a | 0.42±0.01b |
| BA 0.1 + NAA 0.01 | 95 | 39.66±0.57a | 4.23±0.25b | 0.433±0.05ab | 10.66±1.15c | 0.81±0.05a |
| BA 0.1 + IBA 0.01 | 95 | 28.66±2.30b | 4.76±1.10b | 0.433±0.05ab | 15.33±2.08b | 0.47±0.05b |
| BA 0.1 + 2.4-D 0.01 | 95 | 37.66±2.51a | 4.43±1.00b | 0.333±0.05b | 8.33±0.57c | 0.48±0.01b |
Table 2.
Characteristics of in vitro growth by 12 weeks TDZ and auxin treatments on MS medium in cell lines of Imperata cylindrica ‘Rubra’ cultured for 5-year
|
PGRsz treatment (㎎/L) |
REy (%) |
Shoot no./ Calli cluster |
Shoot length (㎝) |
Fresh weight (g) |
Adventitious root number |
Adventitious root length (㎝)x |
| TDZ 2.0 + IAA 0.01 | 100 | 27.33±2.51a | 3.83±0.28b | 0.40±0.00b | 16.00±1.00b | 0.70±0.03a |
| TDZ 2.0 + NAA 0.01 | 100 | 17.66±0.57b | 5.50±0.50a | 0.43±0.11b | 16.33±1.52b | 0.49±0.00c |
| TDZ 2.0 + IBA 0.01 | 100 | 14.66±2.88b | 5.90±0.17a | 0.43±0.11b | 21.00±0.00a | 0.67±0.02b |
| TDZ 2.0 + 2.4-D 0.01 | 100 | 17.66±0.57b | 4.83±1.04ab | 0.53±0.05a | 17.66±1.15b | 0.48±0.36c |
Table 3.
Characteristics of in vitro growth by 12 weeks auxin treatments on MS medium in cell lines of Imperata cylindrica ‘Rubra’ cultured for 5-year
|
PGRsz treatment (㎎/L) |
REy (%) |
Shoot no./ Calli cluster |
Shoot length (㎝) |
Fresh weight (g) |
Adventitious root number |
Adventitious root length (㎝)x |
| MS | 52 | 5.66±2.08b | 5.66±0.20b | 1.03±0.15a | 26.66±1.52c | 0.11±0.00d |
| MS + NAA 0.01 | 90 | 9.00±2.64b | 7.16±0.40a | 0.76±0.11b | 49.00±1.00a | 0.33±0.02a |
| MS + IBA 0.01 | 47 | 11.66±2.08b | 5.70±0.10b | 0.73±0.11b | 43.33±1.52b | 0.28±0.03b |
| MS + 2.4-D 0.01 | 32 | 28.66±6.02a | 4.26±0.46c | 0.60±0.10b | 9.00±1.00d | 0.18±0.01c |
Table 4.
Characteristics of in vitro growth by sucrose concentration on MS mediun containing 0.1 ㎎/L BA and 0.1 ㎎/L 2,4-D for 12 weeks culture in cell lines of Imperata cylindrica ‘Rubra’ cultured for 5-year
| Sucrose concentration |
REz (%) |
Shoot no./ Calli cluster |
Shoot length (㎝) |
Fresh weight (g) |
Adventitious root number |
Adventitious root length (㎝)y |
| 1.5% | 100 | 8.33±1.52b | 4.40±0.20b | 0.23±0.05b | 14.66±1.52d | 0.45±0.02d |
| 3.0% | 92 | 26.66±6.50a | 5.63±0.90a | 0.56±0.05b | 26.00±1.732c | 0.51±0.0c |
| 6.0% | 76 | 23.00±2.64a | 6.60±0.52a | 2.70±0.60a | 48.66±2.309b | 0.79±0.00a |
| 9.0% | 92 | 13.66±4.04b | 6.70±0.60a | 2.83±0.76a | 54.00±1.732a | 0.63±0.01b |
Sucrose treatment
Various concentration of sucrose was treated on MS medium containing 1.5%, 3.0%, 6.0% and 9.0%, respectively.
Acclimatization of regenerants
Rooted plantlets were transplanted into pot after adaptation at culture bottle and field with optimal conditions. As shown in Fig. 5 and Fig. 6, acclimatization of regenerants by percentage opening area (%) of culture-bottle cap, 0%, 5%, 25%, 50% and 100% for 1 week, respectively.
Flow Cytometry Analysis
The flow cytometry analysis was performed according to the manufacture’s protocol, the CyFlow Ploidy Analyser (CyFlow Cube 6, Sysmex, Germany) to analyze polyploidy of the regenerants and the leaves of I. cylindrica ‘Rubra’ plants. Young leaf tissue samples of approximately 0.5 ㎠ were extracted from each treated seedling, chopped with a blade in 200 μL of extraction buffer (CyStain® UV Precise P, Sysmex-Partec, Goerlitz, Germany), and then filtered through a 30 µm mesh filter (CyStain® UV Precise P, Sysmex-Partec, Goerlitz, Germany) to remove debris. Afterward, 800 μL of DAPI staining solution (CyStain® UV Precise P, Sysmex-Partec, Goerlitz, Germany) was added.
Statistical analysis
All of the experiments were executed with three replication. Using the SAS program (SAS, 9.2, Institute Inc, USA), statistical analysis was conducted by Duncan’s multiple range test (DMRT, p=0.05). Frequency and percentage were used to analysis the qualitative characters whereas mean and standard deviation were used for quantitative data analysis.
Results and Discussion
Effect of plant growth regulators on adventitious buds (shoots) of long-term cultured cell lines
Because MS medium containing 0.1 ㎎/L of BA and 0.1 ㎎/L of 2,4-D was used in adventitious buds (shoots) induction in our primary study, we tested MS medium containing 0.1 ㎎/L BA combined with 0.01 ㎎/L of various auxins the in long-term cultured cell lines. Hence, the regeneration ability of long-term cultured cell lines, with effect of plant growth regulators combination on shootings of long-term cultured calli, was investigated firstly (Table 1, Table 2). As shown Table 1 and Fig. 1, 0.1 ㎎/L BA combining 0.01 ㎎/L auxins showed 95% to 100% regeneration. Shoot number per calli cluster and fresh weight were the highest on MS medium containing 0.1 ㎎/L BA and 0.01 ㎎/L NAA. Adventitious root number, adventitious root length and shoot length were the highest on MS medium containing 0.1 ㎎/L BA and 0.01 ㎎/L IAA.
In addition, we checked effect of a kinds of powerful shooting plant growth regulator, TDZ and auxins combination on organogenesis in long-term cultured cell lines. As shown Table 2 and Fig. 2, 2.0 ㎎/L TDZ combining 0.01 ㎎/L auxins showed 100% regeneration. Shoot number per calli cluster and fresh weight were the highest on MS medium containing 2.0 ㎎/L TDZ and 0.01 ㎎/L IAA. Adventitious root number and shoot length were the highest on MS medium containing 2.0 ㎎/L TDZ and 0.01 ㎎/L IBA. Comparing 0.1 ㎎/L BA treatment (Table 1) and 2.0 ㎎/L TDZ treatment (Table 2), regeneration (%) was higher in 2.0 ㎎/L TDZ treatment, but shoot number and adventitious root number were higher in 0.1 ㎎/L BA treatment.
As a kinds of cytokinin, BA and TDZ are major plant growth regulators in shoot induction of in vitro culture of plant tissues (Chai et al., 2011; Cho and Byeon, 2011; Goh et al., 2011; Ryu et al., 2013a, 2013b). In this study, an efficiency of regeneration was not significantly different between BA and TDZ. Effect of TDZ on organogenesis was appeared not strong compared with BA in long-term culture of I. cylindrica ‘Rubra’. The reason might be resulted in a kind of acclimatization at the cell level for five-year culture in vitro. Various kinds of cytokinin and auxin treatments should be investigated to obtain a more detail understanding of organogensis patterns in further study (Kamińska and Sliwinska, 2023; Murashige and Skoog, 1962; Ryu et al., 2013a, 2013b; Shigeki et al., 2009). Organogenesis pattern with cytokinin treatment in this study was coincident with other researches in long-term culturing with cell lines (Chai et al., 2011; Yang et al., 1999).
Effect of plant growth regulators on adventitious roots of long-term cultured cell lines
In order to evaluate regeneration ability of long-term cultured cell lines, effect of plant growth regulators on adventitious roots with long-term cultured calli was investigated (Table 3, Fig. 3). As shown Table 3, regeneration (%) was 32% to 90% by kinds of auxins (NAA, IBA and 2,4-D). Among the auxins treated on MS medium containing 0.1 ㎎/L NAA on MS medium showed 90% of regeneration. Shoot number per callus cluster was the highest on MS medium containing 0.01 ㎎/L NAA. Adventitious root number, shoot length and fresh weight were the highest on MS medium containing 0.01 ㎎/L IBA.
Generally, IAA, IBA, and NAA are used for adventitious root induction, while 2,4-D is imployed for callus induction and callus proliferation (Chai et al., 2011; Fatiha et al., 2019; White, 1984; Yang et al., 1999). As types of auxins, a low concentration of IBA, NAA and 2,4-D induced adventitious roots, but significant difference was not observed among the types of auxins. Therefore, various concentration of auxins should be investigated in order to obtain more detailed understanding of adventitious roots in further study.
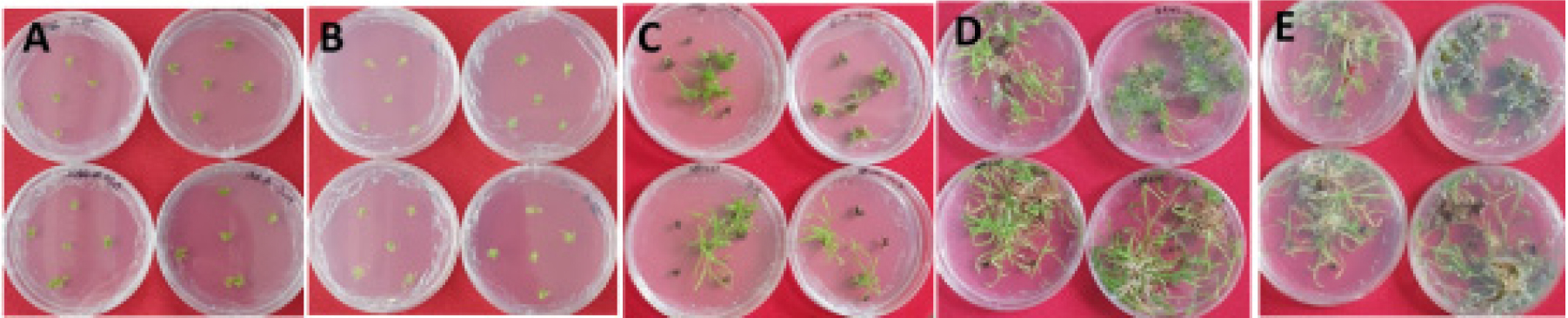
Fig. 3.
Plates for effect of 0.01 ㎎/L auxins on adventitious roots formation. A&B: 0 Day after culture, the face (A) and Back (B), C: 4 weeks after culture, D & E: 12 weeks after culture, the face (D) and Back (E). Auxins were shown in Table 3.
Effect of sucrose concentration on organogenesis of long-term cultured cell lines
Because the regenerants with green and red leaves (RGR) were become brown early compared with regenerants with all green leaves (RAG) in same culture condition, different sucrose concentration were treated on MS medium containing 0.1 ㎎/L BA and 0.1 ㎎/L 2.4-D (Table 4, Fig. 4). As a result of sucrose concentration treatment regeneration was shown from 76% to 100%, respectively. Shoot number per callus cluster was the highest on MS medium containing 3% sucrose. Adventitious roots and adventitious buds were higher on MS medium containing 6% and 9% sucrose. Especially, RGRs were mainly selected on medium supplement with 9% sucrose (Fig. 4C).
It has reported that plastid mutant has weak ability of photosynthesis generally. RGR has also demonstrated a weakness in photosynthetic function, as it exhibited a browning effect over a shorter culture period compared to RAG. For a more detailed understanding of RGR culture and effective RGR selection, various conditions related to photosynthesis, such as the types and concentrations of carbohydrates, should be examined (Kamińska and Sliwinska, 2023; Lee et al., 2023c; Murashige and Skoog, 1962).
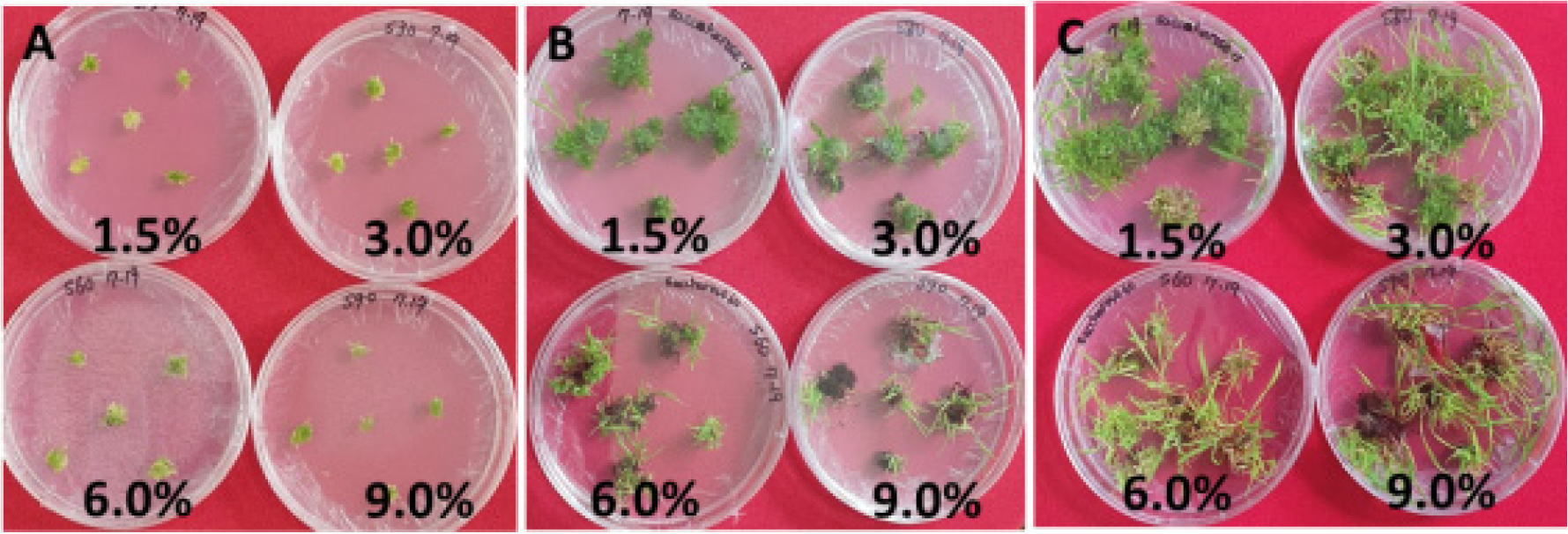
Fig. 4.
Plates for effect of sucrose concentration (%) on in vitro growth. A: 0 Day after culture, B: 4 weeks after culture, C: 12 weeks after culture. Sucrose concentration(%) were shown in Table 4.
Acclimatization of regenerants
In order to an efficient acclimatization survival (%) of the rooted regenerants by percentage opening area (%) of culture-bottle cap was investigated. As shown Fig. 5, survival (%) of the rooted regenerants was different by opening area (%) of culture-bottle cap, and 0% opening area of culture-bottle cap was the best survival condition. The acclimation steps were proceeded by one-week incubation in bottle for one-week (Fig. 6A), on sterilized soil of culture-bottle without opening the cap for one-week (Fig. 6B) and opening of culture-bottle cap after one-week incubation (Fig. 6C). Finally the rooted plantlets were transferred into pot (Fig. 6D) and field (Fig. 6E) successfully.
Acclimation rate is different from plant species, in general (Kang et al.. 2021; Ryu et al., 2013a). Thus, optimal acclimation condition also must be settled down by each plant species. Based on one-week closing of bottle cap, an efficient acclimation was established on the five-year cultured cell lines in this study.
Flow cytometry of regenerants and calli derived from long-term cultured cell lines
Regenerants and calli derived from different plant growth regulators in in vitro cultured Japanese blood grass were analyzed by flow cytometry. As a result of flow cytometry analysis (Fig. 7), regenerants derived from PGRs treatment (Fig. 7B, C, D, E) and calli (Fig. 7F) showed some differences at ploidy level compared with mother plant (control). Interestingly, ploidy peak of 4× was more strong on 0.1 ㎎/L 2,4-D treatment (Fig. 7B, 7C, 7F) compared with 0.01 ㎎/L 2,4-D treatment (Fig. 7D, 7F).
Flow cytometry is a simple, rapid and economical method for ploidy detection of plants (Cousin et al., 2009; Jin et al., 2008; Liu et al., 2020; Meng and Finn, 2002). Ploidy level changes are related to somaclonal variation induced by plant growth regulators during long-term in vitro culture in various kinds of plant species (Cousin et al., 2009; Dewir et al., 2018; Ferreira et al., 2023; Garcia et al., 2019; Jin et al., 2008; Meng and Finn, 2002; Tomaszewska et al., 2021). Our results showed minor changes but resulted in 2,4-D treatment. This is in consistent with other researches with plant growth regulators treatment (Dewir et al., 2018; Garcia et al., 2019).
In conclusion, we confirmed the stable and sustainable regeneration ability, identified optimal acclimation conditions, and evaluated the genetic stability of regenerants and callus in five-year cultured cell lines of I. cylindrica ‘Rubra’. The results imply that sustainability of long-term culturing of cell lines in an in vitro system provides important cues for the mass production of Poaceae over an extended period, serving as a valuable plant resources.









