Introduction
Materials and Methods
Preparation of plant material
Cryopreservation using droplet-vitrification
Regrowth assessment and plant regeneration
Statistical analysis
Results and discussion
Effect of preculture treatment on regrowth rate (%) of cryopreserved shoot tips of strawberry cultivars
Effects of loading and dehydration conditions on regrowth rate (%) from cryopreserved shoot tips of strawberry cultivars
Effects of post-culture media with various growth regulators on regrowth rate (%) of cryopreserved shoot tips of strawberry
Introduction
Cryopreservation is an important tool for long-term conservation of plant genetic resources for enhancement of vegetatively propagated plants and their improvement in agriculture (Gonzalez-Benito et al., 2004; Medina et al., 2007; Coste et al., 2015). Strawberry is one of the important nutritious fruit crops in many countries and is commonly preserved as plants either in field or in insect proof screen houses (Niino et al., 2003). It is not always possible to preserve the crop under natural conditions due to possible risks of losses caused by biological and natural hazards. Cryopreservation has been studied as a viable procedure for the long-term conservation of strawberry germplasm (Clavero-Ramırez et al., 2005). Several cryopreservation protocols have already been developed for this species (Hirai et al., 1998; Reed and Hummer, 1995). There are more than 200 accessions of strawberry cultivars in Korea (Rousseau-Gueutin et al., 2009; Lim et al., 2016). For strawberry preservation, there always exists a possibility of contamination by runners of disease-infected plants under natural conditions. Therefore, previously, Reed and Hummer (1995) developed an alternative method termed as in vitro preservation of strawberry cultures at low temperature for medium term storage. Cryopreservation techniques are now advanced to a stage where they can be implemented for practical storage of germplasm (Reed, 2001). Among the vitrification-based cryopreservation techniques, droplet vitrification has demonstrated high recovery and established as an efficient method for many plant species using various explants, including shoot tips (Sakai and Engelmann, 2007; Benelli et al., 2013). Although there exists a few reports on strawberry cryopreservation (Reed and Hummer, 1995; Wu et al., 1997; Hirai et al., 1998), there are no reports on application on a large-scale involving germplasm lines.
Vitrification involves treatment of samples with highly concentrated vitrification solution to induce dehydration of the cells, which minimizes the probability of intracellular ice formation during freezing in liquid nitrogen (Fahy et al., 1984). Cryopreservation is an alternative for the storage of plant germplasm at an ultra-low temperature (-196℃) in liquid nitrogen. At this temperature, cell divisions and all other biological activities are completely arrested in such a way that the viability of the stored material is retained, so that their biological functions and growth can be reactivated after thawing and transfer to the regrowth medium (Towill, 1991). In addition, vitrification procedures offer greater potential for the preservation of plant tissues with unique requirements for freeze- induced dehydration (Steponkus et al., 1992). The addition of highly concentrated cryoprotectant solution to tissues initiates the formation of amorphous glass at ultra-low temperatures (-196℃) and prevents formation of ice crystals during freezing. The vitrification method has become the preferred method for cryopreservation of plant material from several species (Sakai, 2000; Sakai et al., 2002). The method is widely used for cryopreservation of shoot tips of various plant species (Engelmann, 1997). The main objective of the present study was to develop an easy and efficient method of cryopreservation via droplet-vitrification for shoot tips of the two cultivars of strawberry ‘Wonkyo3114’ and ‘Gurumi40’ and to achieve high recovery of regrowth rate from cryopreserved explants.
Materials and Methods
Preparation of plant material
In vitro grown strawberry (Fragaria × ananassa Duch.) cvs., ‘Wonkyo3114’ and ‘Gurumi40’ were used in this study after multiplication from runner tips. In vitro stock shoots were maintained in shoot multiplication medium composed of Murashige and Skoog (MS; 1962) medium supplemented with 2.0㎎/L 6-benzylaminopurine (BA) + 0.1 ㎎/L 1- naphthaleneacetic acid (NAA) and 30 g/L sucrose + 2.6 g/L phytagel (Duchefa Biochemie B.V., The Netherlands). The pH of the medium was adjusted to 5.8 before autoclaving at 121℃ for 15 min. The stock cultures were maintained at 24 ± 1℃ under a 16 h photoperiod with a light intensity of 50 µmol s-1m-2 provided by cool-white fluorescent tubes. Subculturing was done at every 4 weeks intervals. Explants used for experiments were excised from 6 weeks old plants (Fig. 1A).

Fig. 1.
Cryopreservation of shoot tips of Strawberry (Fragaria × ananassa) by droplet-vitrification. (a) Source of in vitro grown strawberry plantlets used for shoot tips collections. (b) Position of the shoot tips on the plantlets (marked in red color) from which shoot tips are excised. (c) The excised shoot tips used for cryopreservation experiment. (d) Shoot tips are treated with loading solution (LS, C4) for 40 min at 25oC. (e) Dehydrated with PVS3 (B5) for 60 min. (f) Dehydration of shoot tips in 2.5µL PVS3 droplets carried on sterilized aluminum foil strips. (g) Immersion of aluminum foil along with shoot tips into the liquid nitrogen (LN) for 1 h. (h) Cryopreserved shoot tips were unloaded in MS+0.8M sucrose for 40 min at 35oC for thawing. (i) Inoculating the cryopreserved shoot tips on post-thaw culture medium containing NH4NO3-free MS medium with GA3 1.0 + BA 0.5 for 5 weeks and later cultured on MS medium with BA 0.5 for 9 weeks.
Cryopreservation using droplet-vitrification
Shoot tips (~1- 2 ㎜ in length, Fig. 1C) were excised from 8 weeks old in vitro grown plantlets (Fig. 1B) using sterile forceps and scalpel under a stereo microscope under aseptic conditions (Fig. 1C). The cultures were cold-hardened in dark at 4℃ for various time periods ranging from 0 to 6 weeks and precultured in MS medium containing 0.3 - 0.5 M sucrose. The cultures were incubated for different time durations (30 and 40 h) at 25℃. The various preculture treatments are as follows: MS + 0.3 M Sucrose for 30 h, MS + 0.3 M Sucrose for 40 h, MS + 0.5 M Sucrose for 30 h, and MS + 0.5 M Sucrose for 40 h. Precultured shoot tips were osmoprotected with a loading solution (LS, C4) for 40 min. Loading solution (LS, C4) (Kim et al., 2009b) comprised of MS medium containing 35% of PVS3 (17.5% of glycerol + 17.5% of sucrose) without DMSO. Apparently, the loaded shoot tips were exposed to dehydration solution (B5) (Kim et al., 2009a) containing 80% of PVS3 (40% of glycerol + 40% of sucrose) for 60 min at 25℃. The shoot tips were transferred onto droplets containing 2.5 µL PVS3, that were placed on sterilized aluminum foil strip (4.0 ㎝ × 0.5 ㎝, Fig. 1E), followed by direct immersion in LN (Fig. 1F). The shoot tips were removed from the LN after 30 min and transferred into 2 ml cryotubes filled with LN for cryostorage for 1 h. Aluminum foils with shoot tips were removed from LN and immediately placed into an unloading solution containing liquid MS with 0.8 M sucrose at room temperature for 40 min.
Regrowth assessment and plant regeneration
The cryopreserved, unloaded shoot tips were post-thaw cultured in Petri dishes (90 ㎜ in diameter) for shoot recovery in the presence of different post-cultured media under standard conditions, as described by Lee et al. (2011) and Wang et al. (2014). Rewarmed shoot tips were post-cultured in NH4NO3-free MS medium containing 3% sucrose + 1.0 g/L casein + 1.0 ㎎/L GA3 + 0.5 ㎎/L BA for shoot recovery until 5 weeks followed by transferring to normal MS medium containing 3% sucrose + 1.0 g/L casein + 0.5 ㎎/L GA3 until 9 weeks. Subsequently, the plantlets were transferred to hormone free-MS medium. After attaining the height of 8 - 10 ㎝, the plantlets were transferred to greenhouse for hardening. Filter-sterilized GA3 was added to the medium after autoclaving, while BA was directly added to the medium before autoclaving. The cultures were placed at 24 ± 1℃ in the dark for 1 day and then transferred to light conditions for recovery. All the cultures were grown in petri dishes for 5 weeks, and later cultured in bottles. Shoot regrowth was defined as percentage of shoot tips regenerating into shoots with the development of leaves after 4-6 weeks of post-thaw culture. Development of shoots along with roots was observed after 8 weeks in hormone-free MS medium. Consequently, fully in vitro grown plantlets were transferred to soil under greenhouse conditions for acclimatization.
Statistical analysis
In cryopreservation experiments, shoot tips receiving all treatments but without freezing in LN served as the treated control (−LN), while cryopreserved (+LN) shoot tips served as test material. At least 10-12 shoot tips (replicates) were used for each treatment. All the experiments were conducted twice. Results are presented as means with their standard error (SE). The data were analyzed (mean ± SE) from two experiments, and least significant differences (LSD) were calculated at P < 0.05.
Results and discussion
Effect of preculture treatment on regrowth rate (%) of cryopreserved shoot tips of strawberry cultivars
The sucrose concentration and time duration of preculture treatments had no significant effect on the regrowth rate (%) of treated control (−LN) of the shoot tips in both the cultivars (‘Wonkyo3114’ and ‘Gurumi40’); however, significant effect was observed on the regrowth rate of cryopreserved (+LN) shoot tips of the two cultivars of strawberry (Table. 1). The regrowth rate (%) was higher from the shoot tips of the treated control (−LN) than from the cryopreserved (+LN) shoot tips in both the cultivars. For cryopreserved (+LN) shoot tips, the highest regeneration rate (%) in both the cultivars was obtained when the shoot tips were exposed to the preculture treatment (MS + 0.3 M sucrose for 40 h at 25℃). On the other hand, upon exposure of the shoot tips to increased concentration of sucrose (0.5 M), a decrease in the regrowth rate (%) was observed, thus demonstrating that standardization of sucrose concentration plays an important role during preculture treatment of the explants for cryopreservation protocol. The highest regrowth rates (%) were 75.3% and 48.5% for cryopreserved shoot tips of ‘Wonkyo3114’ and ‘Gurumi40’, respectively. The regrowth rate (%) was lower for cryopreserved (+LN) shoot tips before preculture treatment compared with the regrowth rate after preculture treatments. On the contrary, the preculture treatment had no influence on the regrowth rate (%) in treated control (−LN) in both the cultivars.
Table 1. Effect of preculture treatment on regrowth rates of strawberry shoot tips before (‒LN) and after cryopreservation (+LN)
Exposure of in vitro grown explants to preculture treatment in the presence of various sucrose concentrations is known to enhance the survival rate (%) from cryopreserved shoot tips. Previous reports by various researchers have displayed the effect of various sucrose concentrations on regrowth rate (%) during preculturing which acts as supporting evidence to our research. Yamamoto et al. (2012) employed 0.8 M sucrose in preculture treatment for 48 h for strawberry shoot tips, and Niino et al. (2003) observed difference in the shoot regrowth during the preculture of strawberry shoot tips at various concentration of sucrose in the preculture medium. Similarly, Wang et al. (2014) reported that in Chrysanthemummorifolium, the highest shoot regrowth rate (75%) was obtained when shoot tips were precultured with 0.5 M sucrose for 24 h. Both lower (0.25 M) and higher (0.75 M) sucrose concentration resulted in marked reduction in shoot regrowth rate. On the other hand, our results were in agreement with the results reported by Pinker et al. (2009) in their study on strawberry using droplet-vitrification method, which displayed that the highest recovery rate (60%) was achieved after preculture with 0.25 M sucrose for 24 h. These reports showed that sucrose concentration and incubation plays an important role in regrowth of shoots. The employed sucrose concentrations and incubation periods in this study were based on our recent experiments with C. morifolium (Yi et al., 2018a).
Effects of loading and dehydration conditions on regrowth rate (%) from cryopreserved shoot tips of strawberry cultivars
The regrowth rate (%) was observed at a minimum level from cryopreserved (+LN) shoot tips when they were not treated with loading solution, but the regrowth rate (%) was high in treated control (−LN) in both the cultivars of strawberry (Table. 2). The regrowth rate (%) ranged from 62 to 76 in treated control (−LN). When the shoot tips were treated with loading solution (LS, C4) containing 35% of PVS3 (17.5% of glycerol + 17.5% of sucrose) for 40 min followed by exposure to dehydration solution (B5) containing 80% of PVS3 (40% of glycerol + 40% of sucrose) for 40 min at 25℃, prior to direct immersion in LN for 1 h, the regrowth rates (%) were 55.6 and 50.5 for the cultivars ‘Wonkyo3114’ and ‘Gurumi40’, respectively. Regrowth rates (%) started to decrease when the exposure time exceeded 40 min in the dehydration solution (B5). The optimum time duration of exposure to LS (C4) and dehydration solution (B5) was 40 min for regrowth of cryopreserved (+LN) shoot tips from both the cultivars of strawberry.
Table 2. Effect of loading and dehydration solutions and exposure time on the regrowth rate of strawberry shoot tips before (‒LN) and after liquid nitrogen exposure (+LN)
Treatment of the shoot tips with the loading solution (LS) for the purpose of osmoprotection is important to induce tolerance towards dehydration (Sakai et al., 2008; Yamamoto et al., 2012) and selection of appropriate LS is important, as it is highly sensitive to cryotoxicity. The sucrose concentration of the LS varies from species to species (Karen and Kartha 2009; Yamamoto et al., 2011). This method has been successfully used for the cryopreservation of certain plant species such as Jerusalem artichoke (Zhang et al., 2017), blueberry (Wang et al., 2017), citrus (Yi et al., 2018b), sweet potato (Park and Kim, 2005), including strawberry (Pinker et al., 2009). For successful cryopreservation, droplet-vitrification seems to be a controlled procedure for dehydration and prevention of injury from chemical toxicity or excessive osmotic stresses during the treatment with PVS2. Interestingly, in our study, the vitrification solution (B5) was devoid of toxic substances such as dimethyl sulfoxide (DMSO) and ethylene glycol (EG). Exposure of the explants to the dehydration solution (B5) determines the extent of cell dehydration. In support to our results, previous reports by Wang et al. (2014) and Sakai et al. (2008) have proved that the optimal length of exposure to vitrification solution and temperature conditions varies considerably from species to species.
Effects of post-culture media with various growth regulators on regrowth rate (%) of cryopreserved shoot tips of strawberry
In this study, we investigated whether the ammonium ion concentration in post-thaw culture medium had an impact on the viability of cryopreserved strawberry shoot tips. The viability of cooled samples, followed by culturing on NH4NO3-free MS medium containing 3% sucrose, 1.0 g/L casein, 1.0 ㎎/L GA3, and 0.5 ㎎/L BA for first 5-weeks was recorded as 65.7% and 60.5% for the cultivars ‘Wonkyo3114’ and ‘Gurumi40’, respectively (Fig. 2A). However, the regrowth rate (%) of non-treated control in both the cultivars (‘Wonkyo3114’ and ‘Gurumi40’) was not affected. When the shoot tips were transferred to the MS medium containing 0.5 mg/L BA, the highest regrowth rate (%) was recorded as 58.5% and 54.7% for the cultivars ‘Wonkyo3114’ and ‘Gurumi40’, respectively after 9 weeks (Fig. 2B). For cryopreserved (+LN) shoot tips, the lowest regrowth rates (%) were 12.6 % and 10.7 % for the cultivars ‘Wonkyo3114’ and ‘Gurumi40’ in the hormone-free MS medium, respectively. These results reveal that GA3 plays a significant role in ascertaining the best optimal regrowth rate in normal MS medium for both the cultivars of strawberry. The complete plantlets (Fig. 2H) obtained from regenerated shoot tips were transferred to the potting soil (Fig. 1I) under greenhouse conditions for their successful acclimatization.
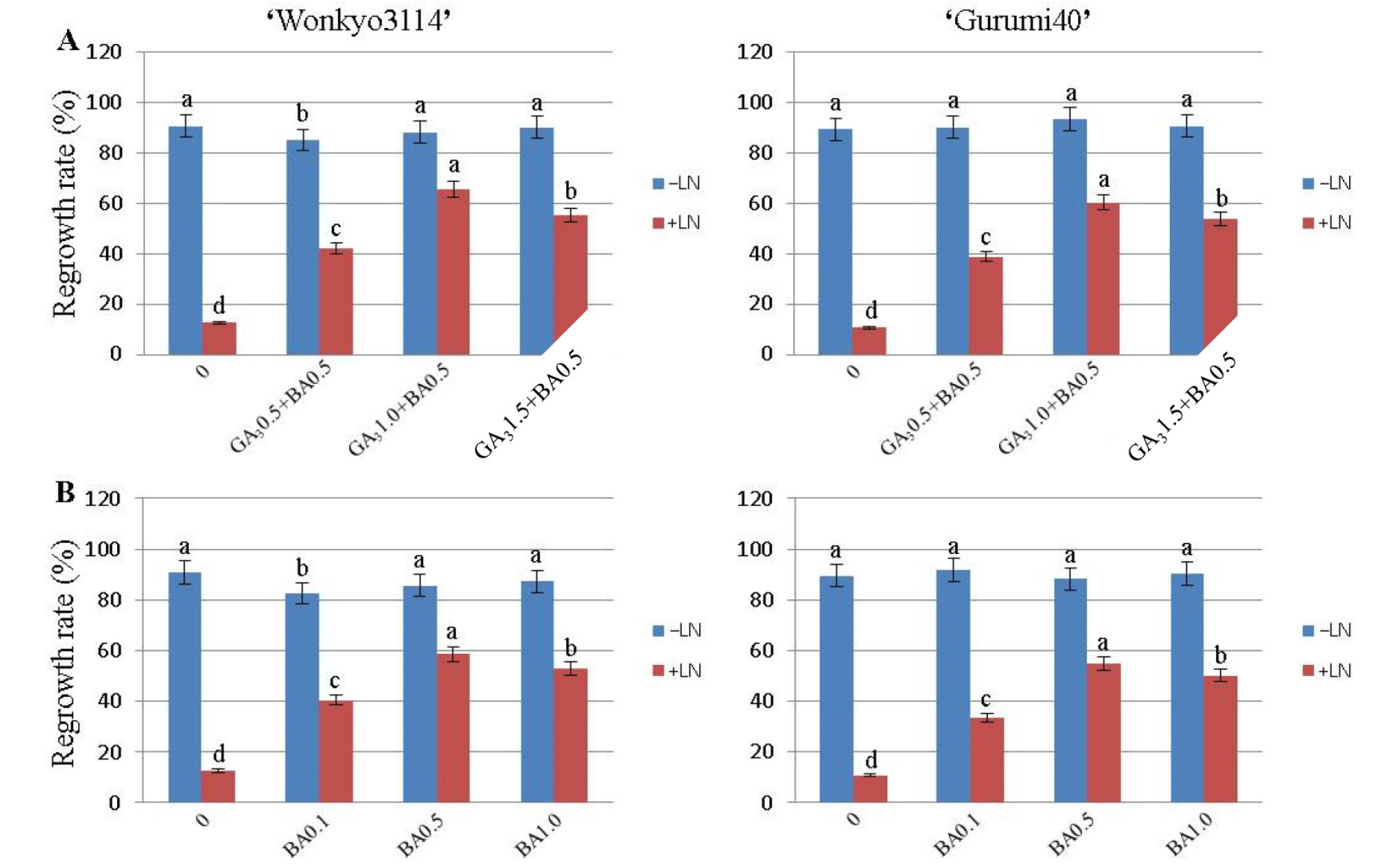
Fig. 2.
Effects of post-culture media containing GA3 and BA on regrowth rate (%) of the treated control (−LN) and cryopreserved (+LN) shoot tips of the two cultivars of strawberry (‘Wonkyo3114’ and ‘Gurumi40’) by droplet-vitrification. Shoot tips excised from 8 weeks old in vitro grown cultures were precultured with MS+0.3M Sucrose for 30 h. Precultured and loaded shoot tips were exposed to loading solution (LS, C4) for 40 min and dehydration solution (B5) for 60 min prior to a direct immersion in LN. Cryopreserved shoot tips were unloaded in MS+0.8M sucrose for 40 min for at 35oC for thawing. A) Rewarmed shoot tips were post-cultured on NH4NO3-free MS medium containing 3% sucrose + 1.0 g/L casein + 1.0 ㎎/L GA3 + 0.5 ㎎/L BA for shoot recovery until 5 weeks. B) Shoot tips later transferred to normal MS medium containing 3% sucrose + 1.0 g/L casein + 0.5 ㎎/L GA3 until 9 weeks. And then the plantlets were transferred to hormone free-MS medium. The plantlets after attaining 8-10 cm height were transferred to greenhouse conditions for acclimatization. The regrowth rate (%) of the cryopreserved shoot tips was recorded 30 d after incubation at 25oC. Each treatment contained 15 replicates. The results are presented as means ± SE. Bars with the same letters are not different from each other statistically according to the least significant difference (LSD) (P = 0.05).
Post-culture medium plays a key role in the recovery of survival of shoot tips after cryopreservation (Wang et al., 2003; Kim et al., 2012). Types and concentration of cytokinins contained in the recovery medium were reported to influence recovery of cryopreserved shoot tips in various plant species (Lambardi et al., 2000; Sant et al., 2008; Li et al., 2015). In our study, cryopreserved strawberry shoot tips were post-thaw cultured in MS medium containing 2.0㎎/L BAP to achieve better recovery. On the other hand, Lambardi et al. (2000) reported that post-thaw survival of white poplar shoot tips was markedly decreased when cryopreserved shoot tips were cultured in hormone-free medium and a significant enhancement was noted in the medium containing GA3. Our results from post-culture treatment of cryopreserved shoot tips are similar to the results reported by Niino et al. (2003), where thawed shoot tips were post-cultured in MS medium containing 0.2㎎/L BA and cultured under standard conditions. Consequently, the results establish droplet-vitrification method as a very practical cryopreservation method for strawberry germplasm and these are proposed as a promising method for cryopreservation of other plants with slight modifications. Further study is necessary to confirm the genetic stability of regenerated plantlets by biochemical and molecular analyses.




