Introduction
Materials and Methods
Preparation of plant material
Cryopreservation using droplet-vitrification
Regrowth assessment and plant regeneration
Statistical analysis
Results and Discussion
Effects of preculture conditions on the regeneration rate (%) of cryopreserved Chrysanthemum cultivars
Effects of loading and dehydration conditions on regeneration rate (%) from cryopreserved chrysanthemum cultivars
Effects of post-culture media on regeneration rate (%) of cryopreserved shoot tips of C. morifolium
Introduction
Chrysanthemum (Chrysanthemum morifolium (Ramat.) from Asteraceae family is one of the most important ornamental crops in the world (Teixeira da Silva, 2004). Chrysanthemum is being used for different purposes such as using these flowers in decorations during various festivals, making flower gardens, and making bouquets for honoring people. It is an important ornamental pot plant or cut flower in Korea (Liang et al., 2014). In Asian countries like China, Japan and Korea, chrysanthemum has been used as medicines since a long time, mainly due to the presence of various biologically active compounds and essential oils (Schwinn et al., 1994; Luyen et al., 2015).
Characterization of chrysanthemums is vital for their conservation for understanding their genetic relationships (Khaing et al., 2013). In addition to ornamental effects, chrysanthemums are well known for their medicinal values, especially, its flowers have numerous antibiotic activities, and therefore chrysanthemum flowers have been used in traditional Chinese medicine for centuries (Yeung, 1983). In addition to its medicinal properties, chrysanthemum extract can also be used as raw material in dye and tea production. Due to over exploitation of these species, the protection of genetic resources are gaining importance to conserve the plant material through various means to meet the future demand (Wang et al., 2014). Therefore, storage of explants at ultra-low temperatures, under liquid nitrogen (LN, -196℃), is being recognized as an ideal means for the long-term conservation of plant germplasm, including ornamental species (Kulus and Zalewska, 2014; Teixeira da Silva et al., 2015).
Previously, various cryopreservation protocols such as preculture-desiccation (Hitmi et al., 2000), encapsulation–dehydration (Martín et al., 2011; Sakai and Engelmann 2007; Yi et al. 2017), vitrification (Martín and González-Benito, 2005), and droplet-vitrification (Kim et al., 2010; Lee et al., 2011) have been reported. Chrysanthemum has been cryopreserved by controlled-freezing rate long before by Fukai et al. (1991). In general, meristematic organs are used for the long-term storage due to their higher genetic stability (Vidal et al., 2005). Previous studies have reported the successful cryopreservation of shoot tips derived from greenhouse grown chrysanthemum plants (Fukai et al., 1990, 1991). On the other hand, studies with C. morifolium by encapsulation-dehydration were rather unsatisfactory, resulting in a low survival and regrowth through callus (Halmagyi et al., 2004; Martin and Gonzalez-Benito, 2009).
Droplet-vitrification was developed more recently and proved to be a suitable protocol for cryopreservation of a wide-range of genotypes within the same species (Panis et al., 2005; Kim et al., 2006; Kim et al., 2012). It is considered very promising for implementing large-scale cryopreservation (Teixeira da Silva et al., 2015). Similarly, regrowth following cryopreservation is most concerned issues in cryopreservation. A droplet-vitrification procedure produced the highest post-cryopreservation regeneration in comparison with other procedures applied for chrysanthemum shoot tips (Lee et al., 2011a,b). However, a droplet-vitrification cryopreservation for diverse Chrysanthemum genotypes has not yet been reported for the establishment of cryo-banking of germplasm (Martín and González-Benito, 2009). The main objective of the present study was to develop an easy and efficient droplet-vitrification cryopreservation for shoot tips of the two cultivars: ‘Borami’ and ‘Yes morning’ of C. morifolium, and to achieve a high recovery of regrowth following cryopreservation of explants.
Materials and Methods
Preparation of plant material
In vitro grown Chrysanthemum (Chrysanthemum morifolium (Ramat.) cvs., ‘Borami’ and ‘Yes morning’ were used in this study. In vitro stock shoots were maintained on shoot multiplication medium composed of Murashige and Skoog (MS, 1962) medium supplemented with 1.0 ㎎/L BA + 0.1 ㎎/ℓ NAA, 30 g/ℓ sucrose + 2.6 g/ℓ phytagel (Duchefa Biochemie B.V., The Netherlands). The pH of the medium was adjusted to 5.8 before autoclaving at 121℃ for 15 min. The stock cultures were maintained at 24 ± 1℃ under a 16-h photoperiod with a light intensity of 50 µmol s-1m-2 provided by cool-white fluorescent tubes. Sub-culturing was done for every 6-7 weeks intervals. Explants used for experiments were excised from 6 week-old plants (Fig. 2a).
Cryopreservation using droplet-vitrification
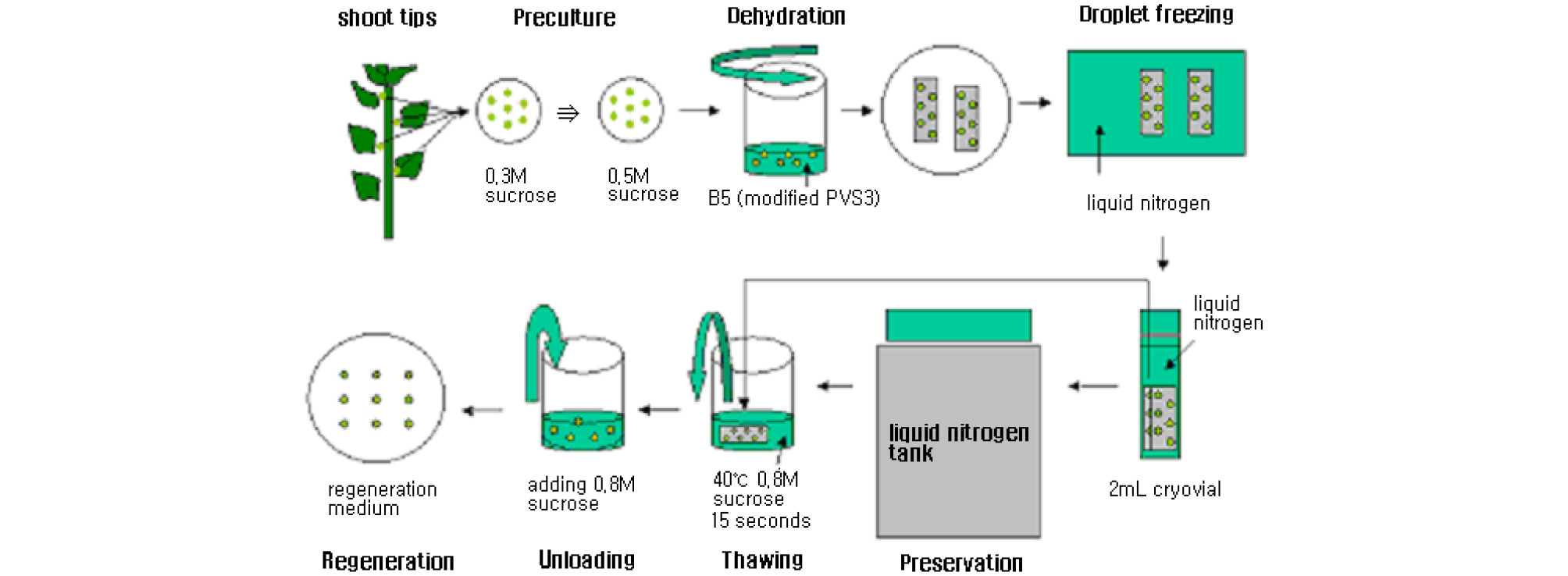
Nodal segments (~0.5 ㎝ in length), were excised from 4-week-old stock shoots and cultured on shoot multiplication medium (Fig. 2b) composed of Murashige & Skoog (MS) medium supplemented with 1.0 ㎎/ℓ BA + 0.1 ㎎/ℓ NAA, 30 g/ℓ sucrose + 2.6 g/ℓ phytagel. The pH of the medium was adjusted to 5.8 before autoclaving at 121℃ for 15 min. The cultures were cold-hardened in the dark at 4℃ for various time periods ranging from 0 to 6 weeks. Shoot tips (1.5–2.0 ㎜ in length) containing 4~5 leaf primordia (Fig. 2c and d) were excised from the shoots that had been cold-hardened for 3 weeks, and precultured on MS medium containing sucrose at 0.3 - 0.7 M concentrations and cultures were incubated for different time durations at 25℃. Droplet-vitrification protocol is explained very clearly in a schematic diagram (Fig. 1). The various preculture treatments (PCT) are as follows: MS + 0.3 M Sucrose for 30 h (PCT-1), MS + 0.3 M Sucrose for 30 h and then treated in MS + 0.5 M sucrose for 16 h (PCT-2), MS + 0.3 M Sucrose for 30 h, and treated in MS + 0.5 M sucrose for 16 h and then treated in MS + 0.7 M Sucrose for 16 h (PCT-3). Precultured shoot tips were osmoprotected with a loading solution (LS) for different duration of time ranging from 0 to 60 min. Loading solution (LS, C6) (Kim et al., 2009a) is composed of MS medium containing 40% of PVS3 (20% of Glycerol + 20% of sucrose) for different time durations ranging from 0 to 60 min. Thus loaded shoot tips were exposed to dehydration solution (B5) (Kim et al., 2009b) containing 80% of PVS3 (40% of Glycerol + 40% of sucrose) for time durations ranging from 0 to 90 min at 25℃. The shoot tips were transferred onto droplets containing 2.5 ㎕ PVS3, each carried on sterilized aluminum foil strip (4.0 ㎝ × 0.7 ㎝) (Fig. 2e). PVS3 is composed of MS supplemented with 50% Glycerol and 50% Sucrose, followed by direct immersion in LN (Fig. 2f). The shoot tips were taken out from the LN after a few minutes and transferred into a 2 ㎖ cryotube filled with LN for cryostorage for 1 h. Frozen foils with shoot tips were taken out from LN and immediately placed into an unloading solution containing liquid MS with 1.2 M sucrose at room temperature for 20 min.

Fig. 2.
Cryopreservation of shoot tips of C. morifolium L. cv. ‘Borami’ by droplet-vitrification. (a) In vitro grown plantlets of Chrysanthemum used for cryopreservation experiments. (b) Nodal explants cultured on MS medium for the emergence of shoot tips. Enlarged form of nodal explant (indicated by arrow). (c) Appearance of shoot tip from nodal axially bud (shoot tip is rounded by circle). (d) dissected shoot tip used for cryopreservation. (e) Dehydration of shoot tips in vitrification solution (B5) droplets on sterilized aluminum foil strips. (f) Immersion of aluminum foil along with shoot tips into the liquid nitrogen (LN) for 1 h. (g) ) Plant regeneration from cryopreserved shoot tips 8 weeks after inoculation. (h) A shoot regenerated into whole plant from cryopreserved shoot tips that had been cultured on post-culture medium (PCM-2) containing NH4NO3-free MS medium. (i) Acclimatized plantlet from cryopreserved (+LN) shoot tips (right), and treated control (−LN) shoot tips (left) under greenhouse conditions. c, d, e) Bar = 0.5 ㎝ ; a, b, f, g, h, i) Bar = 1.0 ㎝.
Regrowth assessment and plant regeneration
Based on our preliminary studies, cryopreserved, unloaded shoot tips were then post-thaw cultured in Petri dishes (90 ㎜ in diameter) for shoot recovery on various post-cultured media (PCM) under standard conditions, as described by Lee et al (2011a) and Wang et al. (2014). Various PCM are as follows: PCM-1: Normal MS medium; PCM-2: NH4NO3-free MS medium; PCM-3: MS + 1.0 ㎎/ℓ 6-benzylaminopurine (BA) + 0.1㎎/ℓ α-naphthalene acetic acid (NAA); PCM-4: MS + 1.0 ㎎/ℓ BA + 0.2 ㎎/ℓ NAA; PCM-5: MS + 0.1 ㎎/ℓ indole-3-acetic acid (IAA) + 0.2 ㎎/ℓ zeatin + 0.05 ㎎/ℓ gibberellic acid (GA3). Filter-sterilized GA3 was added to the medium after autoclaving, while BA and NAA were directly added to the medium before autoclaving. The cultures were placed at 24 ± 1℃ in the dark for 1 day and then transferred to light conditions for recovery. After two weeks of growth, all the plantlets were transferred to normal MS media. Shoot regrowth was defined as percentage of shoot tips regenerating into shoots with development of leaves after 4~6 weeks of post-thaw culture. Shoots developed with roots after 4 weeks onto hormone-free ½ MS medium. Thus, fully in vitro grown plantlets were transferred to soil under greenhouse conditions for acclimatization.
Statistical analysis
In cryopreservation experiments, shoot tips receiving all treatments but without freezing in LN served as the treated control (-LN), while cryopreserved (+LN) shoot tips served as test material. At least 10~12 shoot tips were used in each treatment of three replicates. All experiments were conducted twice. Results are presented as means with their standard error (SE). The data were analyzed (mean ± SE) from two experiments, and least significant differences (LSD) were calculated at P < 0.05.
Results and Discussion
Effects of preculture conditions on the regeneration rate (%) of cryopreserved Chrysanthemum cultivars
The sucrose concentration and time duration in preculture treatments did not significantly affect the regeneration rate (%) of treated control (-LN) of the shoot tips in both the cultivars (‘Borami’ and ‘Yes morning’), while did affect significantly in cryopreserved (+LN) shoot tips of the two cultivars of C. morifolium (Table 1). The regeneration rate (%) was higher from the shoot tips of treated control (-LN) than from cryopreserved (+LN) shoot tips in both the cultivars. For cryopreserved (+LN) shoot tips, the highest regeneration rate (%) was obtained when shoot tips were exposed to the PCT-2 (where exposing shoot tips to MS + 0.3 M Sucrose for 30 h and then treated with MS + 0.5 M sucrose for 16 h) at 25℃ in both the cultivars. On the other hand, when exposing the shoot tips to increased concentration of sucrose (0.7 M), the regeneration rate (%) started decreasing, which showed the standardization of sucrose concentration plays an important role during preculture of the explants for cryopreservation protocol. The highest regeneration rates (%) were 42.1% and 38.8% for cryopreserved shoot tips of ‘Borami’ and ‘Yes morning’, respectively. For cryopreserved (+LN) shoot tips without preculture treatment, the regeneration rate (%) was drastically lower in comparison with the regeneration rate after preculture treatments. However, there was no effect of preculture treatments on regeneration rate (%) in treated control (-LN) in both the cultivars.
Table 1. Effect of preculture treatment on regeneration rates of chrysanthemum shoot tips before (−LN) and after cryopreservation (+LN) following liquid nitrogen immersionz
yThe results are presented as means ± SE. Values in the columns followed by the same letter(s) are not significantly different from each other according to the least significant difference (LSD) (P < 0.05).
Exposure of in vitro grown explants with preculture treatment containing various sucrose concentrations is necessary step to obtain the best shoot regeneration rate (%) from cryopreserved shoot tips. Previous reports by various researchers have displayed the effect of sucrose concentrations on the regeneration rate (%) during preculturing which are supported to our research. Halmagyi et al. (2004) used 0.5 M sucrose in preculture treatment for 24 h, and similarly, Lee et al. (2011a) reported that the highest shoot regrowth was obtained when shoot tips were precultured with 0.3 M sucrose for 31 h. But studies with some other species like gentian (Suzuki et al., 1998) and Lily (Yi et al., 2013) have reported that exposure of explants to sucrose concentration has been also effective for shoot regeneration. The regenerate rates were 42 and 38% in ‘Borami’ and ‘Yes morning’, respectively. In contrast, Halmagyi et al. (2004) reported the plant regeneration rate as 55% after a 10-min incubation in 100% PVS2. On the other hand, our results of preculture treatments in both the cultivars were in corroborative with the results developed by Lee et al. (2011) in Chrysanthemum, and in addition, displays that the explants from non-cold-hardened plants could withstand cryopreservation after preculture in media with increased sucrose concentration.
Effects of loading and dehydration conditions on regeneration rate (%) from cryopreserved chrysanthemum cultivars
The percentage of regeneration rate was observed at a minimum level from cryopresered (+LN) shoot tips when they were not treated with loading solution, but the same was high in treated control (-LN) in both the cultivars of C. morifolium (Table 2). The range of regeneration rate (%) was from 77 to 85 in treated control (-LN). When the shoot tips were treated with loading solution (LS, C6) containing 40% of PVS3 (20% of Glycerol + 20% of sucrose) for 30 min, and followed by exposure to dehydration solution (B5) containing 80% of PVS3 (40% of Glycerol + 40% of sucrose) for 90 min at 25℃, prior to direct immersion in LN for 1 h, the regeneration rates (%) were recorded as 48.1 and 42.8 for the cultivars ‘Borami’ and ‘Yes morning’, respectively. Regeneration rates started decreasing when the exposure time exceeded 90 min in dehydration solution (B5). The optimal time durations of exposure to loading solution (C6) and dehydration solution (B5) were 30 min and 90 min, respectively for regrowth of cryopreserved (+LN) shoot tips from both the cultivars of C. morifolium.
Table 2. Effect of loading and dehydration solutions and exposure time on the regeneration rate of chrysanthemum shoot tips before (−LN) and after liquid nitrogen exposure (+LN)
.
Previously, Kim et al. (2009a, b) developed alternative loading solutions (LSs) and vitrification solutions (VSs) and applied those to Chrysanthemum cv. ‘Peak’, which was sensitive to the cytotoxicity of cryoprotectants used in the droplet-vitrification method. This method has been successfully used for the cryopreservation of certain plant species such as Prunus (De Boucaud et al., 2002), Musa (Panis et al., 2005), including Chrysanthemum (Halmagyi et al., 2004). In studies with alternative LSs, chrysanthemum axillary shoot tips were progressively precultured with various concentrations of sucrose ranging from 0.3 M – 0.7 M with various time durations (Kim et al., 2009a). Interestingly, in our study the vitrification solution (B5) was devoid of toxic substances such as dimethyl sulfoxide (DMSO) and ethylene glycol (EG). During the exposure of explants with the dehydration solution (B5), it determines the extent of cell dehydration. A long duration of vitrification solution exposure was apparently toxic to the cells (Chen et al., 2011). In supportive of our results, previously Panis et al. (2005) stated that the optimal length of exposure to vitrification solution varies from species considerably. In addition, Halmagyi et al. (2004) showed that both full strength and diluted PVS2 were successfully employed for cryopreserving chrysanthemum shoot tips.
Effects of post-culture media on regeneration rate (%) of cryopreserved shoot tips of C. morifolium
In this study, we investigated whether the ammonium ion concentration in post-thaw culture medium (PCM) affects the viability of cryopreserved Chrysanthemum shoot tips. The viability of cooled samples, followed by culturing on NH4NO3- free MS medium for first 5 days was increased to two-fold (80.7%) regrowth rate over those cultured on normal MS medium or MS media containing plant growth regulators. There were no significant differences in regrowth rates among five media used for the treated control (-LN) in two cultivars of C. morifolium (Table 3). For cryopreserved (+LN) shoot tips, the lowest regrowth rates were 42.1% and 38.8% for the cultivars ‘Borami’ and ‘Yes morning’, respectively by normal MS medium (PCM-1; Fig. 2g) while the highest regrowth rates were observed as 80.7% and 75.5% for the cultivars ‘Borami’ and ‘Yes morning’, respectively in NH4NO3-free MS medium (PCM-2). The next highest regeneration rate (%) was obtained by the MS medium supplemented with 1.0 ㎎/ℓ BA + 0.1㎎/ℓ NAA (PCM-3). From our results, Table 3 shown that the best optimal post-culture medium was PCM-3 (NH4NO3-free MS medium) for both the cultivars of C. morifolium. The complete plantlets (Fig. 2h) were obtained from regenerated shoot tips were transferred to the potting soil (Fig. 2i) under greenhouse conditions for their acclimatization.
Table 3. Effects of post-culture media on regeneration rate (%) of the treated control (−LN) and cryopreserved (+LN) shoot tips of the two cultivars of C. morifolium by droplet-vitrification
Previously, Decruse and Seeni (2002) reported the importance of reducing ammonium nitrate in the culture medium to get maximum recovery of cryopreserved shoot tips of Holostemma annulare. Under the long preconditioning treatment, MS medium free of NH4NO3 allowed maximum regeneration rate in LN exposed shoot tips after 45 days of post-freeze culture. Similar results were also observed in the present study with respect to the absence of NH4NO3 in MS medium (Table 3). On the other hand, Kuriyama et al. (1989) found that NH4+ was beneficial for the growth of new rice cells, but it was toxic to cells that had been exposed to LN. Our results from post-culture treatment following cryopreserved shoot tips are most similar to other results developed recently by Wang et al. (2014) in C. morifolium, where thawed shoot tips were post-cultured on MS medium supplemented with 0.05 ㎎/ℓ GA3 in the dark for 3 days, followed by incubating under standard culture conditions. In contrast, studies with C. morifolium by encapsulation-dehydration were not satisfactory, resulting in a low survival and regrowth through callus (Halmagyi et al., 2004), while there was not callus formation from the shoot tips in our report. This result shows droplet-vitrification would be a promising method for cryopreserving Chrysanthemum germplasm. In a conclusion, this study presents a protocol for cryopreservation of Chrysanthemum germplasm resources by droplet-vitrification, and thus it appears that shoot tips of various cultivars of C. morifolium germplasm can be safely conserved by droplet-vitrification method. Further study is necessary to confirm the genetic stability of regenerated plantlets by cytological, biochemical and molecular analyses.