Introduction
Materials and Methods
Reagents
Animals
Preparation of avocado extracts
Scratching behavioral experiment
Induction of AD-Like Skin Lesions and avocado treatment
Evaluation of skin dermatitis severity
Histamine assay
Cytokine Assay
Western blot analysis
Caspase-1 activity
Statistical analysis
Results
Avocado suppress scratching behaviors in mice
Avocado suppress AD symptoms in DNCB-induced AD-like skin lesions
Avocado suppress IgE and histamine serum levels in DNCB-induced AD mice
Avocado suppress the inflammatory cytokines expression in AD-like skin lesions
Avocado suppress the NF-κB activation in AD-like skin lesion
Avocado suppress caspase-1 activation in AD-like skin lesion
Discussion
Introduction
There is increasing evidence suggesting that natural products may represent effective agents in the treatment of various inflammatory diseases (Lee and Kang, 2018). Avocado, one of the superfoods, is beneficial to human health owing to its high levels of nutrients and bioactive phytochemicals. Therefore, avocados have been extensively used in the nutraceutical, cosmetic and pharmaceutical industries. An increasing number of studies have shown that avocados exhibit a range of beneficial effects including anti-oxidation, anti-cancer and hypolipidemic properties (Bhuyan et al., 2016; Lu et al., 2005; Pahua-Ramos et al., 2012). However, the anti-inflammatory mechanisms employed of avocado have not been elucidated.
Skin inflammatory responses are processes that involve the action of multiple factors within a complex network (Hawiger, 2001). Atopic dermatitis (AD) is a common skin inflammatory disease and is associated with disturbances in the skin barrier as well as immune dysregulation. AD is characterized by intense itching, edema, erythema, thickening, severe pruritus and eczematous lesions of the skin (Leung and Bieber, 2003). Genetic, environmental and immune factors have been linked to the pathogenesis of AD (Bieber, 2008). AD is typically treated with corticosteroids (Berke et al., 2012), but long-term treatment can have serious side effects including immunosuppression and epidermal barrier dysfunction (Shiohara et al., 2004). Thus, anti-atopic agents with fewer side effects are needed.
Inflammatory cytokines have been implicated in the initiation and extension of skin inflammation. The symptoms of skin inflammation mainly originate from the response of inflammatory cells to cytokines, this reaction is a pivotal factor in the pathogenesis of skin inflammatory diseases including AD and psoriasis (Nomura et al., 2003). It was reported that TNF-α and IL-6 expression are elevated in AD patients and inhibition of these inflammatory cytokines reduced pathological inflammation (Trefzer et al., 2003)
Nuclear factor-kappa B (NF-κB) is an important regulator of various genes in the immune and inflammatory responses (Tegeder et al., 2001). A number of studies have reported the role of NF-κB in inflammatory diseases (Shin et al., 2019). It was reported that NF-κB activation and the subsequent increase in inflammatory cytokine is important in AD pathology. Additionally, caspase-1, a member of the caspase family, is involved in inflammatory response (Lamkanfi et al., 2004). It has been reported that activated caspase-1 induces the NF-κB activation and regulates inflammatory- related genes expression (Siegmund et al., 2001). Other study has shown that inflammatory mediators were down-regulated by both NF-κB and caspase-1 inhibitors during allergic inflammation (Kim et al., 2011). From this, it was suggested that the NF-κB and caspase-1 pathway could be an ideal target for molecular therapies designed to treat skin inflammation.
Other studies reported that avocado suppress intestine inflammation and reduces the ultraviolet-induced skin inflammation (Rosenblat et al., 2011). Despite the previous studies evidencing the nutritional and pharmacological relevance of avocados, information on the anti-inflammatory mechanism of avocado remain limited. In this study, we investigated the effects of avocado on 2, 4-dinitrochlorobenzene (DNCB)-induced AD symptoms in mice. Moreover, we evaluated the effects of avocado on the expression of inflammatory cytokines as well as activation of NF-κB and caspase-1 in AD-like skin lesions.
Materials and Methods
Reagents
DNCB, compound 48/80, avidin peroxidase (AP), dimethyl sulfoxide (DMSO) and other reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA). ELISA kits for mouse TNF-α/IL-6/IgE were obtained from BD Biosciences. NF-κB antibodies (Abs) were obtained from Santa Cruz Biotechnology (Santa Cruz CA, USA). Caspase-1 colorimetric assay kit was purchased from Biovision (Milpitas, CA, USA).
Animals
Male BALB/c (6 weeks, 19-20 g) and ICR mice (6 weeks, 18~20 g) were purchased from Hyochang Science (Daegu, Korea). Animals were housed at six heads per cage and allowed spontaneous intake of food and water. Animals were kept under a 12-h light/dark cycle at room temperature (24 ± 2℃) and humidity (56 ± 10%). The research was conducted in accordance with the internationally accepted principles for laboratory animal use and care as described in the Daegu Haany university guidelines.
Preparation of avocado extracts
The dried avocado (200 g) was chopped using a blender and then added to 2 L of 70% aqueous ethanol solution at room temperature for 24 h. This extract was filtered and concentrated is a water bath under vacuum, frozen and lyophilized to yield the final ethanol extracts used in this study (yield: 7.31%). When the samples were used, they were dissolved in distilled water and then filtered through a 0.22 ㎛ syringe filter.
Scratching behavioral experiment
Before the experiment, the ICR mice (n=6/each group) were put into acrylic cages (22 × 22 × 24 ㎝) for up to 30 min to allow for acclimation. The behavioral experiments were performed using the method described by Sugimoto et al. (2006). We clipped the rostral area of the skin on the back of each mice and histamine (100 ㎍/㎏) or compound 48/80 (50 ㎍/㎏) was intradermally injected. These scratch inducing agents were dissolved in tween 80 and then used. Control animal received a tween 80 injection. Immediately after the intradermal injection, the mice (one animal/cage) were put back into the same cage for observation. Scratching of the injected site by the hind paws was counted and compared with scratching at other sites, including ears. Each mouse was used only one. The mice generally displayed several scratches for 1 second, and a series of these scratches was counted as one incident in 30 min. Avocado (10 and 100 ㎎/㎏) was administered orally1 h before the scratching agents.
Induction of AD-Like Skin Lesions and avocado treatment
DNCB (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in (3:1 acetone olive oil vehicle) and used as a sensitizer for inducing AD-like skin lesions in mice (Lee et al., 2010). The dorsal skin of BALB/c mice was shaved with depilatory and gauzed one day before sensitization. Mice were randomly divided into 4 groups (n=6/group): vehicle, DNCB, and DNCB plus avocado treatment (10 ㎎/㎏ and 100 ㎎/㎏). Exposed skin was treated with vehicle or 200 μL of a 1% DNCB solution. On day 4 after sensitization, the dorsal skin was challenged with a 0.5% DNCB (200 μL) solution three times per week. This procedure was repeated for 4 weeks and avocado was orally administered every day for 2 weeks.
Evaluation of skin dermatitis severity
The severity of dermatitis was assessed using the Eczema Area and Severity Index scoring system: 0, no symptoms; 1, mild symptoms; 2, moderate symptoms; and 3, severe symptoms. The severity of dermatitis was evaluated by three blinded examiners using the naked eye. The sum of each of their individual scores was defined as the dermatitis score for erythema/hemorrhage, edema, excoriation/erosionand scaling/ dryness (Hanifin et al., 2001).
Histamine assay
The mice were anesthetized with ether following an overnight fast, blood was then drawn and serum obtained by centrifugation. Serum concentrations of histamine were measured using a specialized ELISA kit. We performed this ELISA according to the manufacturer’s instructions (Neogen, Lexington, USA).
Cytokine Assay
TNF-α and IL-6 secretion were measured by modification of an enzyme-linked immunosorbent assay (ELISA) as previously described (Kim et al., 2010). Briefly, 96-well plates were coated with anti- human monoclonal Abs and incubated overnight at 4℃. After washes, sample or a standard solution containing TNF-α and IL-6 was added to each well and incubated for 2 h. Biotinylated anti-mouse Abs were added and incubated for 2 h. After washing, we sequentially added AP and 2,2′-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) substrate containing H2O2. Finally, we evaluated the optical density of the plates at 405 nm using a microplate reader.
Western blot analysis
To analyze the expression level of indicated proteins, nuclear extracts in skin tissue were homogenized and then isolated by Nuclear Extraction Reagents (Pierce Thermo Scientific, Rockford, USA). After bicinchoninic acid protein quantification, the supernatant was mixed with a sample buffer, separated by gel electrophoresis, and transferred to a membrane. The membranes were then blocked with 5% skim milk and subsequently exposed to primary Abs. After washing, membranes were incubated with secondary Abs for 1 h. After washing with 0.1% PBST, protein bands were visualized using an ECL detection system purchased from Pierce Thermo Scientific (Rockford, IL, USA).
Caspase-1 activity
The enzymatic activity of caspase-1 was assayed using a caspase colorimetric assay kit according to the manufacturer’s instructions (R < D systems, Minneapolis, USA). Briefly, the protein supernatant from skin tissue was incubated with 50 μL reaction buffer and 5 μL caspase substrates at 37℃ for 2 h. The absorbance was then measured was measured using a plate reader at a wavelength of 405 ㎚. Equal amounts of total protein from each lysate were quantified using a BCA quantification kit.
Statistical analysis
Results are reported as mean ± S.D and each experiment was completed at least-three times. The results were examined using an independent t- tests and ANOVA with a Tukey post hoc test. P < 0.05 was considered significant.
Results
Avocado suppress scratching behaviors in mice
The anti-scratching effects of avocado were investigated using a compound 48/80 or histamine-induced scratching behavior model. When avocado was orally administered 1 hour prior to histamine or compound 48/80 injections, the scratching behaviors were reduced. The inhibition rate of avocado (100 ㎎/㎏) was approximately 40.01 ± 3.17% and 38.83 ± 4.27%, respectively (Fig. 1).
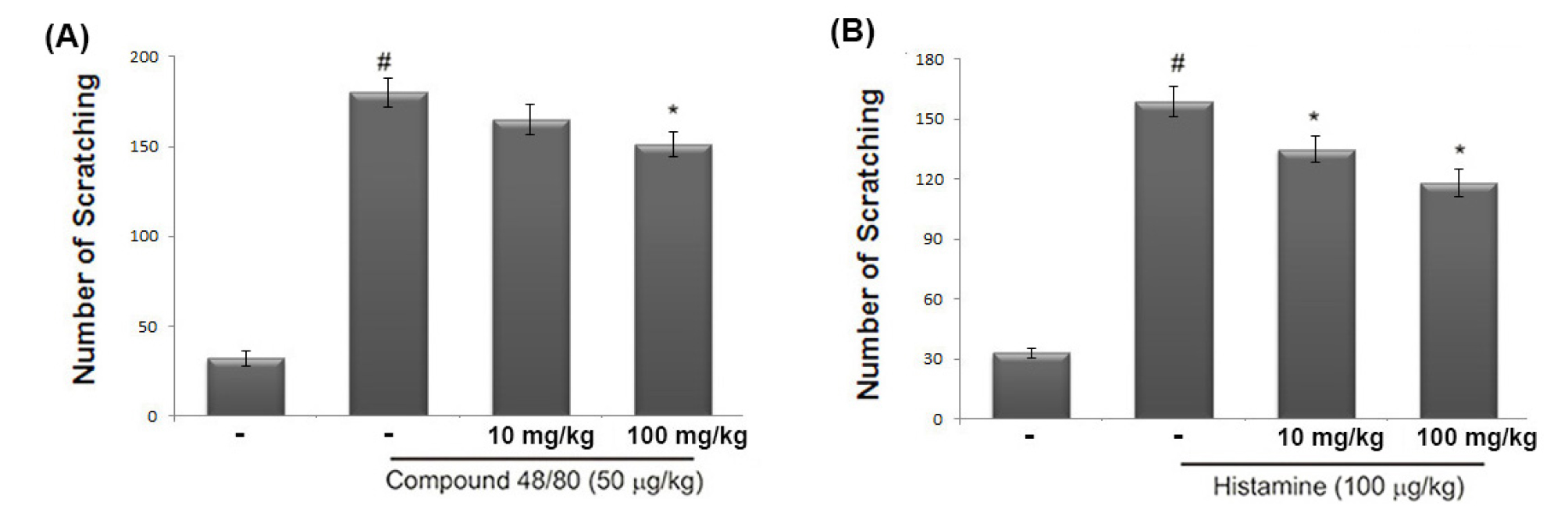
Fig. 1.
Effect of avocado on the scratching behavior in mice. (A and B) The 70% ethanol extract of avocado (10 or 100 ㎎/kg) was orally administered 1 hour before the intradermal injection of compound 48/80 (50 ㎍/kg) or histamine (100 ㎍/kg). Scratching behavior was counted as one incident of scratching for 30 minutes. The data represents the mean ± S.D. of experiments (#P < 0.05; significantly different from vehicle control group, *P < 0.05 vs. compound 48/80 or histamine-treated group).
Avocado suppress AD symptoms in DNCB-induced AD-like skin lesions
To evaluate the beneficial effects of CA in an AD in vivo model, DNCB was applied to BALB/c mice. When the mice received avocado extract for 2 weeks, DNCB-induced AD symptoms such as erythema, edema and dryness were significantly reduced (Fig. 2A). Additionally, we observed that the skin severity scores were significantly lower in the avocado group when compared to those in the DNCB-treated group (Fig. 2B).
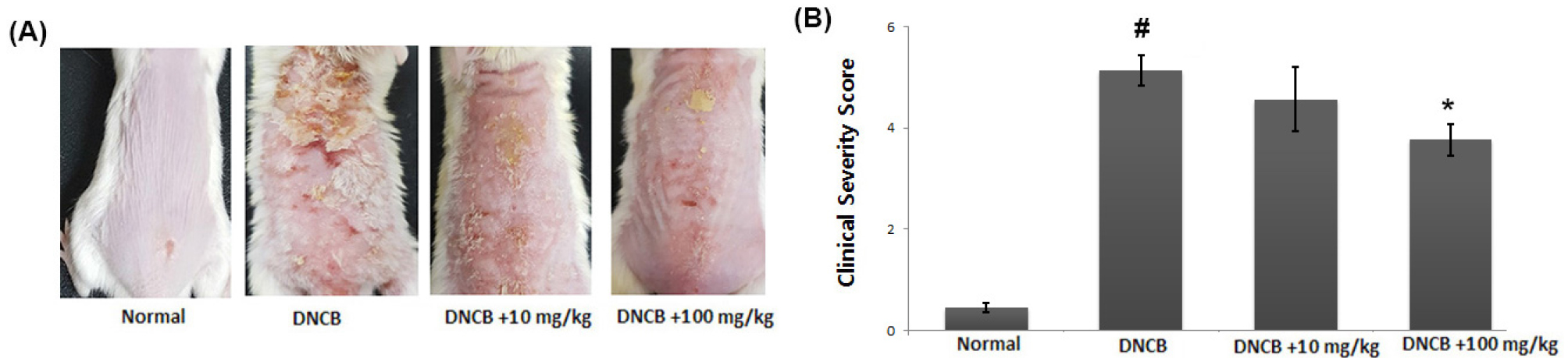
Fig. 2.
Effect of avocado on DNCB-induced AD in mice. (A) Clinical feature of AD-like skin lesions. (B) The score of skin severity is represented. The results are presented as mean ± SD. (#P < 0.05; significantly different from vehicle control group, *P< 0.05; significantly different from DNCB- treated group).
Avocado suppress IgE and histamine serum levels in DNCB-induced AD mice
A crucial feature of AD is the pathological secretion of IgE and histamine (Saeki et al., 2009). Total serum IgE levels are often increased in AD patients and are used as a diagnostic tool and therapeutic target (Gomez, 2019). Thus, we evaluated the effect of avocado on IgE and histamine levels in serum using ELISA. As shown in Fig. 3A and B, application of DNCB resulted in increased release of IgE and histamine. In contrast, the avocado -treated group showed a considerable reduction in serum IgE and histamine. The inhibition rates of IgE and histamine by avocado (100 ㎎/㎏) were approximately 28.9% and 25.3%, respectively (P <0.05).
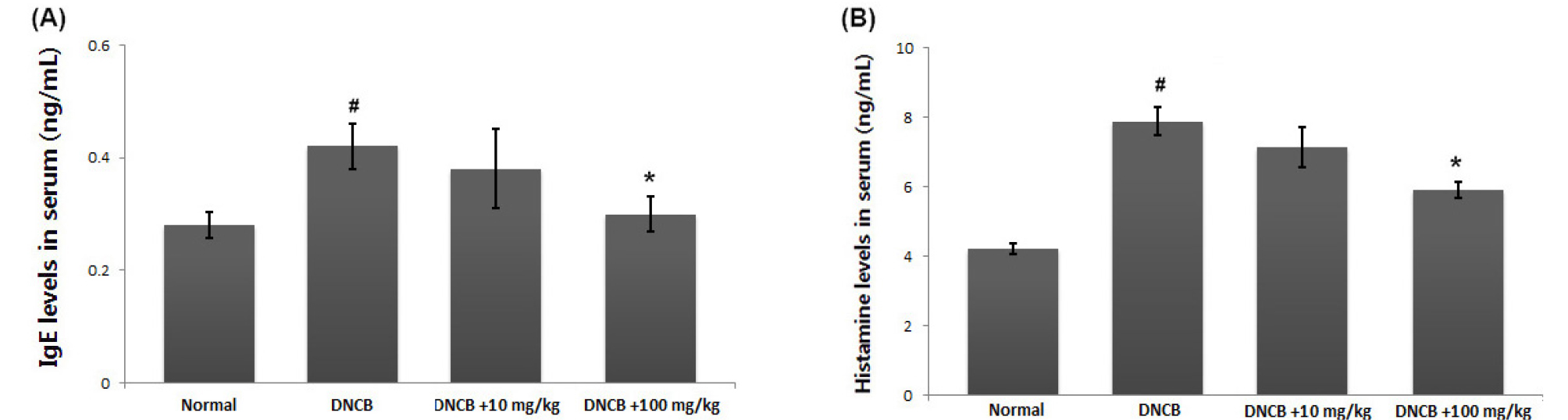
Fig. 3.
Effects of avocado on the IgE and histamine serum levels. (A and B) Blood samples in DNCB-induced AD mice were collected and then levels of serum IgE and histamine were measured using ELISA assay kit assay according to the manufacturer’s directions method. The results are presented as mean ± SD. (#P < 0.05; significantly different from vehicle control group, *P< 0.05; significantly different from DNCB- treated group).
Avocado suppress the inflammatory cytokines expression in AD-like skin lesions
Inhibition of TNF-α or IL-6 levels is one of the most widely accepted treatment strategies for AD (Bunikowski et al., 2001). To investigate the anti-inflammatory activity of avocado, we examined the regulatory effect of avocado on TNF-α and IL-6 levels in the AD-like skin lesion. At the end of the experiment, skin tissues were homogenized and ELISA was performed. Our results show that the levels of TNF-α and IL-6 were significantly increased in skin tissues from DNCB-treated mice compared to that of control. However, administration of avocado reduced these cytokines in DNCB-treated animals. The inhibition rate of TNF-α and IL-6 levels by avocado (100 ㎎/㎏) were approximately 23.7% and 31.8%, respectively (Fig. 4A and B).
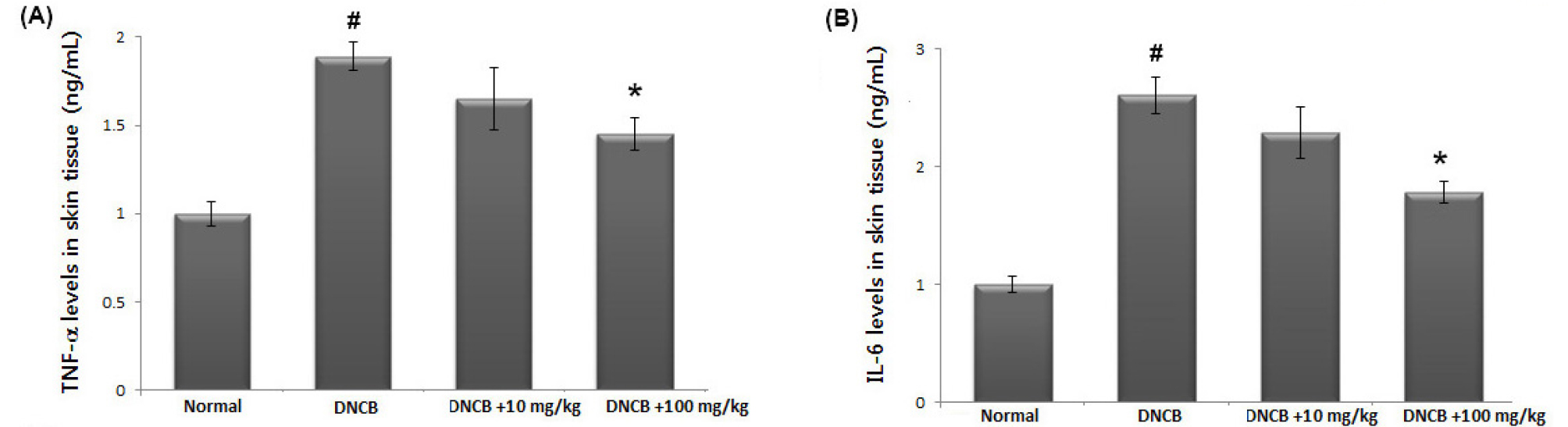
Fig. 4.
The effects of avocado on TNF-α and IL-6 levels in AD-like skin lesion. (A and B) At the end of experiment, the skin tissues were cut out and homogenized. The level of TNF-α and IL-6 in the indicated groups was measured via ELISA. The results are presented as mean ± SD. (#P < 0.05; significantly different from vehicle control group, *P< 0.05; significantly different from DNCB- treated group).
Avocado suppress the NF-κB activation in AD-like skin lesion
As NF-κB activation is associated with the skin inflammatory response, we predicted that the anti-inflammatory mechanism of avocado may be mediated via suppression of NF-κB activation. As activation of NF-κB requires the translocation of NF-κB into the nucleus, we evaluated the effects of avocado on the nuclear pool of NF-κB in AD-like skin lesion. As illustrated in Fig. 5A, we confirmed that the levels of Rel/p65 were increased in the nucleus, while avocado reduced these increased levels in AD-like skin lesions (Fig. 5A). The relative levels of NF-κB (in the nucleus) are shown in Fig. 5B.
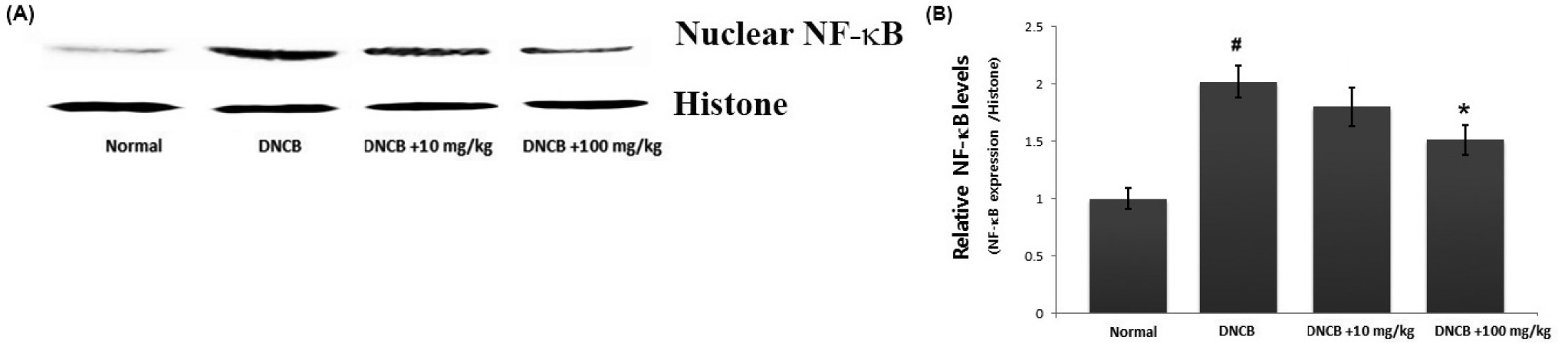
Fig. 5.
The effect of avocado on NF-κB activation in AD-like skin lesion. (A) Nuclear extracts from skin tissue were prepared and the NF-κB levels in nucleus measured via western blot analysis. (B) The relative levels of NF-κB were represented. The results are presented as mean ± SD. (#P < 0.05; significantly different from vehicle control group, *P< 0.05; significantly different from DNCB- treated group).
Avocado suppress caspase-1 activation in AD-like skin lesion
Activation of caspase-1 is associated with the production of inflammatory mediators. To identify the anti-inflammatory mechanism of avocado, we evaluated whether avocado could suppress activation of caspase-1 in AD-like skin lesion. The skin tissues were homogenized and we evaluated the effects of avocado on caspase-1 activation using a caspase-1 assay kit. As shown in Fig. 6, the enhanced caspase-1 activity in DNCB-induced AD-like skin lesion was down-regulated by treatment with avocado in a dose-dependent manner.
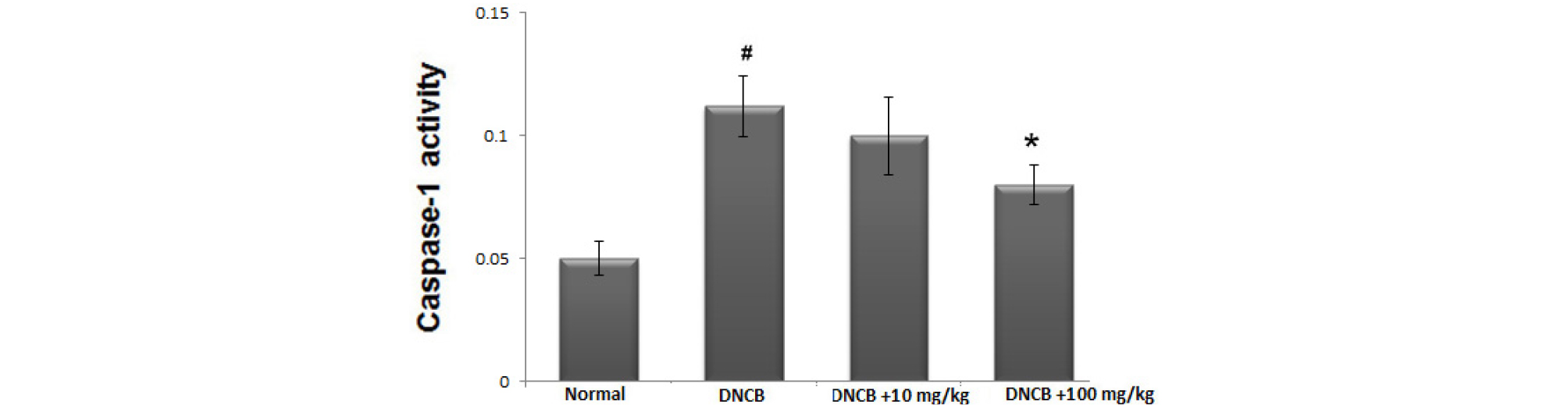
Fig. 6.
The effect of avocado on caspase-1 activation in AD-like skin lesion. At the end of experiment, the skin tissues were cut out and homogenized. The enzymatic activity of caspase-1 in skin tissue was tested by a caspase colorimetric assay. The results are presented as mean ± SD. (#P < 0.05; significantly different from vehicle control group, *P< 0.05; significantly different from DNCB- treated group).
Discussion
Avocados are rich in flavonoids, polyphenols, and phenolic acids and have been reported to have numerous pharmacological activities. Thus, they are a good dietary source of substances with various health benefits. However, the precise mechanisms of these effects remain unclear. In this study, we demonstrate the regulatory mechanism of avocado mediated relief of skin inflammation such as AD. These findings reveal that avocado attenuates the compound 48/80- or histamine-induced scratching behaviors and DNCB-induced AD clinical symptoms in mice. In addition, this anti-inflammatory activity of avocado is mediated through the regulation NF-κB and caspase-1 activation in AD-like skin lesions.
AD is known to result from immune system dysregulation, ultimately resulting in allergic inflammation (Gold and Kemp, 2005). IgE dysregulation has been implicated in the pathogenesis of AD and it was reported that serum IgE concentration is elevated in patients with AD (Allam and Novak, 2006; Brenninkmeijer et al., 2008). Steroid therapy is a crucial factor in the treatment of AD because of its anti-inflammatory activity. However, steroids cannot be administered long-term because of their deleterious side effects (Das and Panda, 2017). Therefore, natural products have gained attention in developing other treatment for AD (Shiohara et al., 2004). In this study, we found that avocado significantly reduced AD symptoms such as itching, erythema, edema and dryness in mice. Additionally, we observed that avocado inhibited the DNCB-induced IgE levels in the serum. In pathological skin conditions, histamine is involved in inducing itching and edema (Minami and Kamei, 2004). It was reported that patients with AD have higher histamine levels compared with those in healthy subjects and treatment of anti-histamine agents ameliorate the AD symptoms (Imaizumi et al., 2003). In this study, we have shown that avocado attenuates DNCB-induced histamine levels in the serum. These results suggest that avocado exerts an anti-atopic effect by regulation of the clinical symptoms of AD.
Accumulated experimental evidence shows that inflammatory cytokines are pivotal factors in the pathogenesis of skin inflammatory diseases. It was also reported that TNF-α and IL-6 levels are elevated in patients with AD and plays an integral role in AD pathogenesis (Fedenko et al., 2011; Wong et al., 2001). Thus, we attempted to investigate whether avocado’s anti-inflammatory activity is exerted by inhibition of these inflammatory cytokines in AD-like skin lesions. Here we show that the expression levels of TNF-α and IL-6 were increased in AD-like skin lesions compared to those in controls and that administration with avocado reduced these enhanced levels of TNF-α and IL-6. Previous other study reported that avocado inhibited the production of NO and inflammatory cytokines in LPS-induced RAW 264.7 macrophage cells (Au et al., 2007). Consistent with these results, we observed that avocado attenuates inflammatory mediators in AD-like skin lesions. From this, we were able to infer that the anti-inflammatory activity of avocado may be associated with the suppression of inflammatory cytokines.
The production of these cytokines is dependent on activation of the transcription regulator NF-κB (Gilmore and Garbati, 2011). In inactive state, complexes of NF-κB/inhibitor of κB (IκB) are sequestered in the cytoplasm. During the inflammatory process, IκB kinase (IKK) complex phosphorylate and degrade the IκB protein and NF-κB is translocated to the nucleus where it can bind to promoter of target genes and activate gene expression. Caspase-1 is a member of the caspase family and plays an important role in apoptosis and inflammation (Bouchier-Hayes and Martin, 2004). Activation of caspase-1 is associated with an increase of inflammatory mediators such as cytokines.It was reportedthat caspase-1 deficiency in mice reduced the cytokine production (Wang et al., 2005). Additionally, it has been showed that the activation of caspasse-1 induces NF-κB and MAPK-signaling pathways. Therefore, to identify the anti-inflammatory mechanism of avocado, we investigated whether avocado could suppress the activation of NF-κB and caspase-1 in AD-like skin lesion. The results demonstrate that avocado inhibited NF-κB translocation into the nucleus and caspase-1 activation. Therefore, we hypothesized that avocado attenuates skin inflammation by blocking NF-κB/ caspase-1 activation in DNCB-induced AD-like skin lesion. Although we evaluated the role of avocado treatment in the attenuation of NF-κB/ caspase-1 activation, the effects of avocado on other pathways including MAPK-signaling were not investigated. Therefore, further studies are needed to more precisely evaluate the role of avocado in the inhibition of the skin inflammatory response.
In conclusion, avocado can reduce clinical symptoms and IgE and histamine serum levels in a DNCB-induced AD model. Additionally, we demonstrated that the anti-inflammatory activities and mechanism of avocado can attributed to the regulation of inflammatory cytokine expression and NF-κB/caspase-1 activation in AD-like skin lesion. These results provide experimental evidence that avocado might be a potential candidate for treating inflammatory skin diseases such as AD.




