Introduction
Materials and Methods
Collection of plant material
Adzuki bean extraction for antioxidant activity
DPPH assay
ABTS assay
Ferric reducing antioxidant power assay
Reducing power assay
SOD assay
Total polyphenol content assay
Analysis of flavonol content in KABL
Analysis of isoflavone content in KABLs
α-Glucosidase inhibition activity assay
Determination of Tyrosinase inhibition activity
Data analysis
Results
The distribution of antioxidant activity in leaf extracts from the 223 KABLs
Selecting adzuki bean landraces with high or low antioxidant activity using RACI
Comparison of flavonoid content between the high and low antioxidant groups
Comparison of α-glucosidase and tyrosinase inhibition activity between the high and low antioxidant groups
Correlations among antioxidant activity, phytochemicals, and α-glucosidase and tyrosinase inhibition activity in adzuki bean extracts
Comparison of antioxidant activity, phytochemicals, and α-glucosidase and tyrosinase inhibition activity between leaf and seed extracts
Discussion
Introduction
The adzuki bean (Vigna angularis L.) is cultivated around the world, mainly in Asiatic countries, as a diverse food source (Gohara et al., 2016; Rho et al., 2003). In China and Korea, the bean has been used to treat diuretic dysfunction and other diseases such as dropsy and beriberi (Gohara et al., 2016; Kim and Chung, 2013; Luo et al., 2016). Adzuki beans leaves are also used as medicine and in side dishes in Korea (Moon et al., 2015). Sikryochanyo, the Korean traditional medical book, describes that adzuki bean leaves help treat and prevent diabetes (Kim and Chung, 2013). Although previous studies reported on characteristics of the adzuki bean such as antioxidant activity and phenolic and flavonoid content (Gohara et al., 2016; Han et al., 2015; Luo et al., 2016; Park et al., 2011; Woo et al., 2016), few study authors have analyzed the bean’s leaves.
Reactive oxygen species (ROS) are oxygen-derived molecules that include the superoxide anion, hydroxyl, peroxyl, alkoxyl, nitric oxide, singlet oxygen, hydrogen peroxide, and hypochlorous acid (Fang et al., 2002). ROS damage cellular constituents such as DNA, proteins, amino acids, and lipids (Zou and ChangSam, 2014), and damage caused by ROS is an important risk factor in the pathogenesis of numerous chronic diseases such as asthma, inflammatory arthropathies, diabetes, Parkinson’s and Alzheimer’s diseases, cancers, and human aging (Chiavaroli et al., 2011). Many have reported the beneficial health effects of antioxidants contained in some food (Kim et al., 2012; Kim et al., 2017; Maynard et al., 2003; Sabe et al., 2012). Antioxidant uptake from food is recommended because the antioxidants in foods are considered to detoxify ROS in the human body (Terashima et al., 2013). Some researchers have reported that some antioxidant compounds are present in bound form in food matrices (Acar et al., 2009; Gökmen et al., 2009; Serpen et al., 2007; Serpen et al., 2008). Although these food-antioxidant complexes are not easily absorbed from the intestine, they are likely important for gastrointestinal tract health (Terashima et al., 2013).
Flavonoids are a general name for a group of chemicals that includes anthocyanins, pro-anthocyanins, flavonols, and isoflavones (Luo et al., 2016). Flavonoids provide color to plants and attract pollinators and seed dispersers. They include antioxidants to protect plants against UV radiation, attract insects, act as signaling molecules to facilitate nitrogen fixation, and defend against bacterial and fungal attack, and their bitter or astringent taste repels birds and other animals (Croteau et al., 2000; Kim and Cha, 2017; Wildmann, 2001; Winkel-Shirley, 2001; Winkel- Shirley, 2002). Several beneficial health properties of dietary flavonoids have been recognized in humans due to their antioxidant and antiproliferative effects, which protect the body from various pathologies such as cancers and cardiovascular and inflammatory diseases (Middleton et al., 2000; Nijveldt et al., 2001).
The aim of this study was to evaluate the potential of adzuki bean leaf extracts, by-products of adzuki bean production, as alternative sources of phytochemicals, antioxidants, antidiabetics, and skin whitening. For this purpose, we analyzed the antioxidant activity, flavonoid content, and α-glucosidase and tyrosinase inhibition activity and compared these with the same activity in adzuki bean seed extracts. This paper is the first attempt to assess the flavonoid content and bioactivity in adzuki bean leaf extracts.
Materials and Methods
Collection of plant material
We obtained 223 Korean adzuki bean landraces (KABLs) from the National Agro-biodiversity Center (http://genebank.rda.go.kr), NAS, Rural Development Administration, Republic of Korea. The KABLs had been cultivated with conventional cultural practices in the NAS experimental field during 2015. Fully matured leaves and seeds of KABL from the plants were dried at room temperature and stored at 4℃.
Adzuki bean extraction for antioxidant activity
We added 100 milligrams of each ground sample to 1 ㎖ of 75% EtOH and sonicated the samples for 1 h. Then, we centrifuged the mixtures at 13,000 rpm for 10 min. We collected the clear supernatants in new tubes and used them for antioxidant activity assays.
DPPH assay
We assessed the extracts’ DPPH radical-scavenging activity using a previously published method with slight modifications (Lee and Lee, 2004). Briefly, we added DPPH solution (150 μl; 150 μM in anhydrous EtOH) to 100 ㎕ of sample solution. The mixture was shaken vigorously and left to stand at 25℃ in the dark for 30 min. We measured absorbance at 517 ㎚ using a spectrophotometer (Epoch; Bio-Tek, Winooski, VT, USA), and the results are expressed as IC50 (㎕ sample volume) values.
ABTS assay
We estimated ABTS radical-scavenging activity using a previously described method with some modifications (Re et al., 1999). Briefly, we generated the ABTS radical cation by adding 7 mM ABTS to 2.45 mM potassium persulfate followed by overnight incubation in the dark at room temperature. We diluted the ABTS cation solution with methanol (MeOH) to obtain absorbance of 0.7 ± 0.02 at 735 ㎚, and we added the diluted radical cation solution (190 ㎕) to 10 ㎕ of sample solution. After 6 min of incubation, we measured absorbance at 735 ㎚ with the spectrophotometer. The results are expressed as ascorbic acid equivalents (ASC) per gram of dry weight (㎎ ASC/g).
Ferric reducing antioxidant power assay
We determined ferric reducing antioxidant power (FRAP) using the method published by (Singh et al., 2012) with minor modifications. Briefly, we prepared the FRAP reagent by mixing 0.1 M acetate buffer at pH 3.6 (100 ㎖), 10 mM tripydyltriazine (TPTZ) solution in 40 mM HCl (10 ㎖), and 20 mM ferric chloride solution (10 ㎖). We prepared the FRAP reagent freshly and warmed it to 37℃, and then we mixed 10 microliters 300 ㎕ reagent and recorded the absorbance at 593 ㎚ after 30 min incubation at 37℃. The results are expressed as ㎎ ASC/g.
Reducing power assay
We determined reducing power using a previously published method with slight modifications (Yen and Duh, 1993). Briefly, we mixed a 0.1 ㎖ aliquot of the extract with 0.5 ㎖ phosphate buffer (0.2 M, pH 6.6) containing 1% K3Fe(CN)6 and incubated the mixture at 50℃ for 20 min. After centrifugation at 200 g for 10 min, we mixed the upper layer (10 ㎕) with 390 ㎕ of 1% ferric chloride. We monitored absorbance at 700 ㎚ using a spectrophotometer, and the results are expressed as ㎎ ASC/g.
SOD assay
We measured the superoxide anion scavenging activity as described by (Robak and Gryglewski, 1988) with some modifications. We generated the superoxide anion radicals in 100 ㎕ of Tris-HCl buffer (16 mM, pH 8.0) containing 0.3 mM nitroblue tetrazolium (NBT), 50 ㎕ NADH (0.936 mM) solution, and 100 ㎕ sample + 50 ㎕ Tris-HCl buffer (16 mM, pH 8.0). We initiated the reaction by adding 50 ㎕ phenazine methosulfate solution to the mixture and incubating at 25℃ for 5 min and then measured the absorbance at 560 ㎚. The results are expressed as IC50 (㎕ sample volume).
Total polyphenol content assay
We measured total polyphenol content using a modified Folin–Ciocalteu method (Waterhouse, 2001). We added Folin–Ciocalteu reagent (100 ㎕) to 100 ㎕ of sample solution and allowed it to react at room temperature for 3 min. After we added 100 ㎕ of 2% sodium carbonate, we incubated the mixture at room temperature for 30 min. We measured absorbance at 750 ㎚ on a spectrophotometer using distilled water as the blank. Total phenolic content was reported as milligrams of gallic acid equivalents (GAE) per gram of dry weight sample (㎎ GAE/g).
Analysis of flavonol content in KABL
We transferred 1 gram of each sample into 5 ㎖ polypropylene tubes and mixed this with 2.5 ㎖ of 80% methanol containing 1.2M hydrochloric acid for hydrolysis. We briefly vortexed the mixture and then incubated it at 80℃ for 2 h with tube inversion for mixing the samples with extract solution at 15- min intervals. After incubation, we centrifuged the samples at 14 000 rpm for 3 min and transferred the supernatant into 2 ㎖ Eppendorf tubes. We collected the supernatant and filtered it using a 0.45 ㎛ syringe filter prior to analysis with an Agilent 1260 Infinity HPLC system (Agilent Technologies, Santa Clara, CA, USA). HPLC conditions were as follows: solvent A, 0.1% TFA/H2O; solvent B, CH3CN; gradient, 20% (B), 20-50% (B) in 5 min, 50-100% (B) in 6, hold at 100% (B) for 1 min, re- equilibrate at 20% (B) for 3 min; column temperature, 30℃; and flow rate, 0.40 ㎖/min. The filter detector was set at 370 ㎚.
Analysis of isoflavone content in KABLs
We added 1 gram of each sample to 2 ㎖ of 80% MeOH and sonicated this for 1 h. We hydrolyzed the sample in each tube using 150 ㎕ of 2N NaOH. After mixing for 10 min, we neutralized the solution with 50 ㎕ of glacial acetic acid and centrifuged the sample at 3,000 rpm for 5 min. We collected the supernatant and filtered it using a 0.45 ㎛ syringe filter prior to analysis with the Agilent 1260. We performed the analysis using a Cosmosil 2.5 Cholester (3.0 ㎜× 75 ㎜, 2.5 ㎛; Nacalai Tesque, Inc., Kyoto, Japan). HPLC conditions were as follows: solvent A, 0.1% TFA/H2O; solvent B, 0.1%TFA/CH3CN; gradient, 10% (B) for 0.35 min, 10-30% (B) in 3.96 min, hold at 30% (B) for 0.36 min, re-equilibrate at 10% (B) for 1.8 min; column temperature, 30℃; and flow rate, 0.58 ㎖/min. The filter detector was set at 254 ㎚.
α-Glucosidase inhibition activity assay
We measured α-glucosidase inhibition activity as described by (Zhang et al., 2015). First we premixed each sample (10 ㎕) with 10 ㎕ α-glucosidase (1.1 U/㎖) for 10 min at 37℃; then we added pAPG/MPA/AuNPs (60 ㎕) into the mixed solution and continuously incubated it for 20 min at 37℃. After that, we added PDBA (30 ㎕) to the resulting mixture to obtain its final concentration of 3.00 mM. Lastly, we allowed the reaction solution to stand at room temperature for 15 min. We recorded the absorbance for each sample using UV-vis spectroscopy with the calculated IC50. The inhibitory ratio (%) was expressed as follows:
Inhibitory ratio (%) = (A650/A520-A’650/A’520)/(A’650/A’520)*100
where A650/A520 was the ratio of the absorbance at 650 ㎚ to that at 520 ㎚ in the presence of both the sample and the enzyme and A’650/A’520 was the ratio of the absorbance at 650 nm to that at 520 ㎚ in the presence of the enzyme. The results are expressed as IC50 (㎕ sample volume).
Determination of Tyrosinase inhibition activity
We determined tyrosinase activity using L-tyrosine as a substrate. First, we mixed 70 ㎕ of L-tyrosine (0.03%) with 70 ㎕ 0.05M PBS (pH 6.8). Then, we added 60 ㎕ of sample and 10 ㎕ of tyrosinase (125U/㎖) solution and incubated this at 37℃ for 10 min. After that, we immediately incubated the solution on ice for 5 min. We monitored absorbance at 475 ㎚ using a spectrophotometer. We calculated tyrosinase inhibition activity as follows:
Inhibition (%) = ((A-B)/A)*100
where A was without sample and B was with sample and substrate. The results are expressed as IC50 (㎕ sample volume).
Data analysis
We used least significant difference (LSD) and correlation analysis to determine differences among the 223 KABLs using SPSS Statistics 20 (SPSS Inc., Chicago, IL, USA). The integration of antioxidant capacity results derived from different chemical methods (Sun and Tanumihardjo 2007) allowed us to calculate the relative antioxidant capacity index (RACI).
Results
The distribution of antioxidant activity in leaf extracts from the 223 KABLs
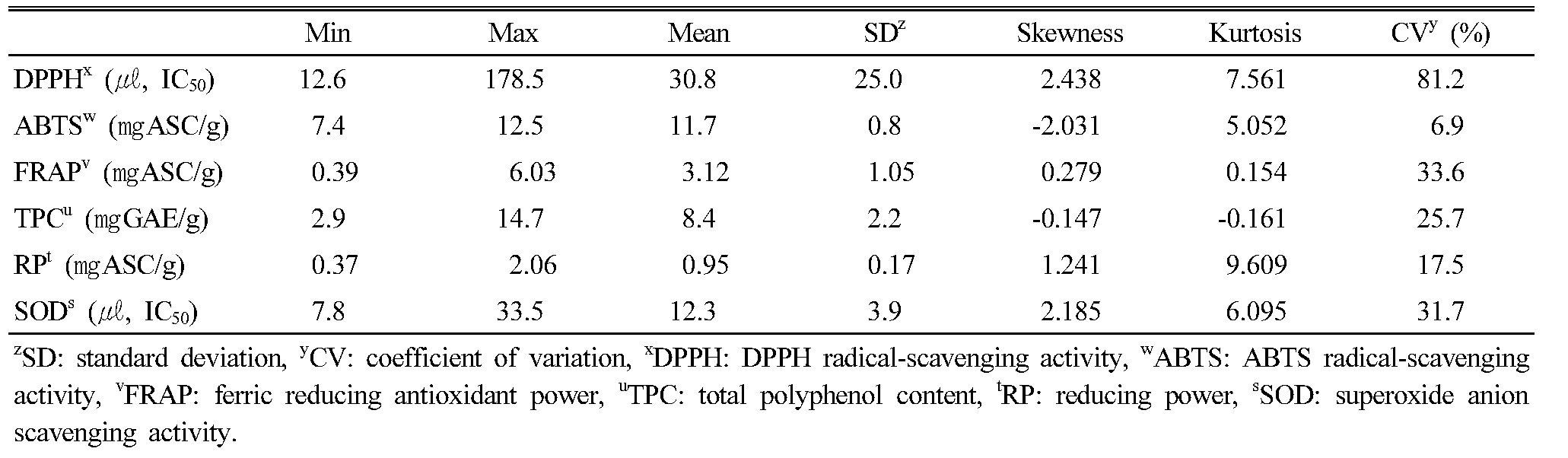
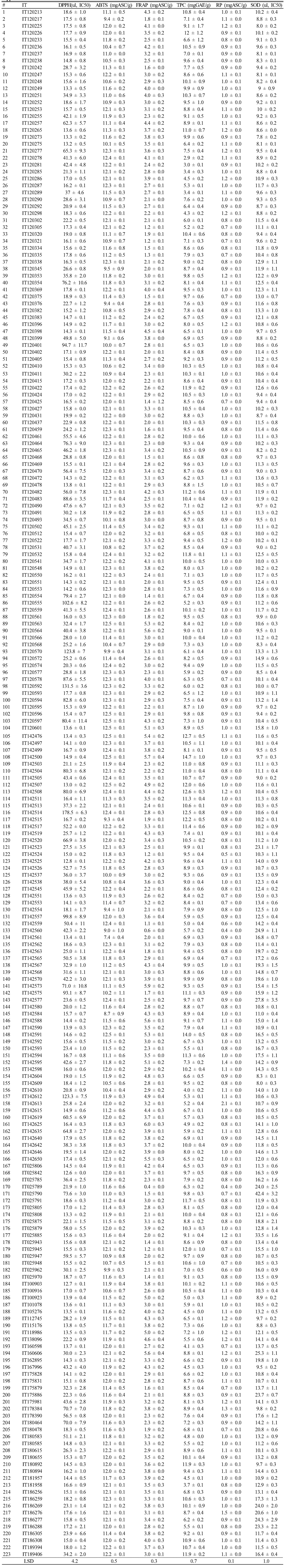
The distribution of antioxidant activity in the leaf extracts of the KABLs is presented in Table 1 and Appendix 1. Among five antioxidant activities, DPPH showed the highest variation (coefficient of variation [CV]: 81.2 %), and ABTS was the lowest (CV: 6.9 %). DPPH and SOD ranged 12.6 to 178.5 and 7.8 to 33.5 (㎕, IC50), respectively. ABTS, FRAP, and RP ranged from 7.4 to 12.5, 0.39 to 6.03, and 0.37 to 2.06 ㎎ ASC/g, respectively, and TPC’s range was 2.9 to 14.7 ㎎ GAE/g.
Table 1. Descriptive statistics for antioxidant activity in the leaf extracts from 223 Korean adzuki bean landraces |
|
Each antioxidant activity assay showed different ranges for the 223 KABLs (Appendix 1): the accessions with the most antioxidant activity were IT025842 in DPPH; IT142500 in ABTS; IT142625 in FRAP; IT142500 in TPC; IT142613 in RP; andIT120273 in SOD, and the accessions with the least activity were IT142514 in DPPH; IT142561 in ABTS; IT025789 in FRAP; IT120278 in TPC; IT025789 in RP; and IT025885 in SOD.
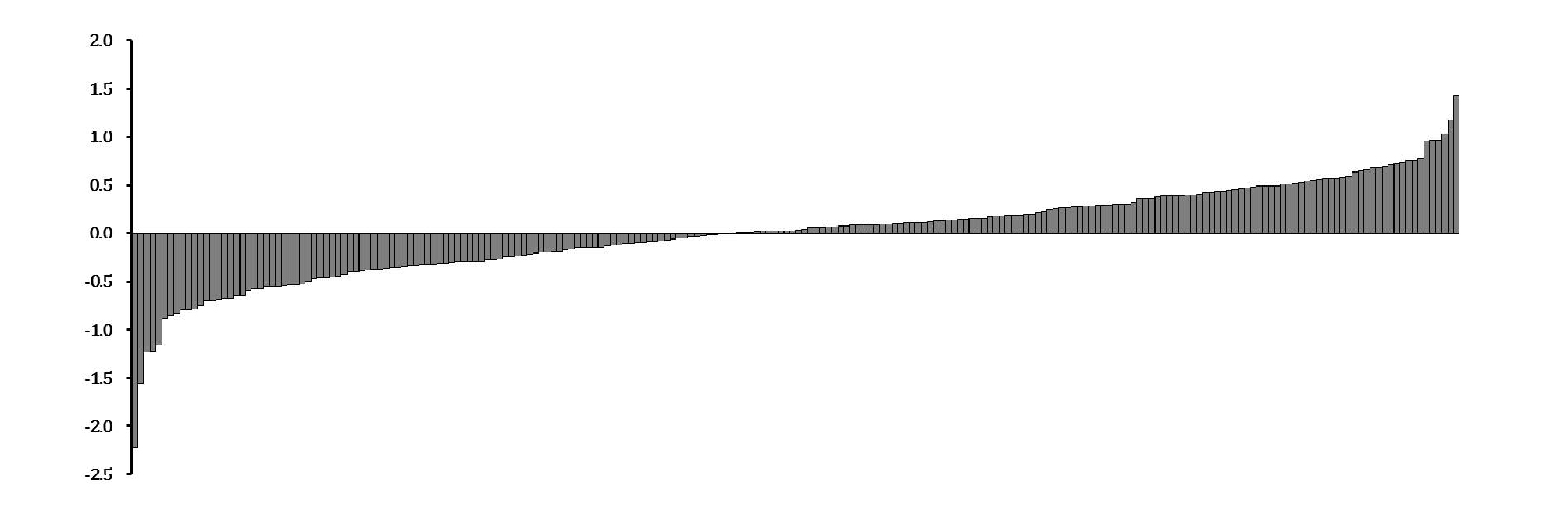
Integrating the antioxidant capacity results derived from different chemical methods allowed us to calculate RACIs, and the results are shown in Fig. 1. We found that IT142500 had the highest RACI, 1.42, followed by IT142476 (1.18), and IT142507 (1.03), with IT142561 having the lowest, -2.23.
Selecting adzuki bean landraces with high or low antioxidant activity using RACI
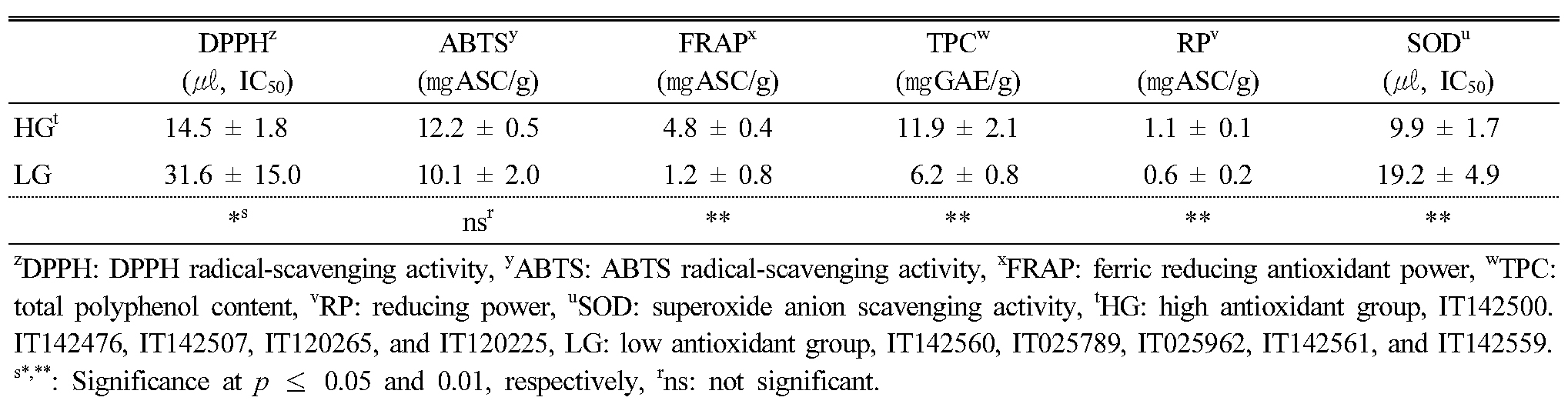
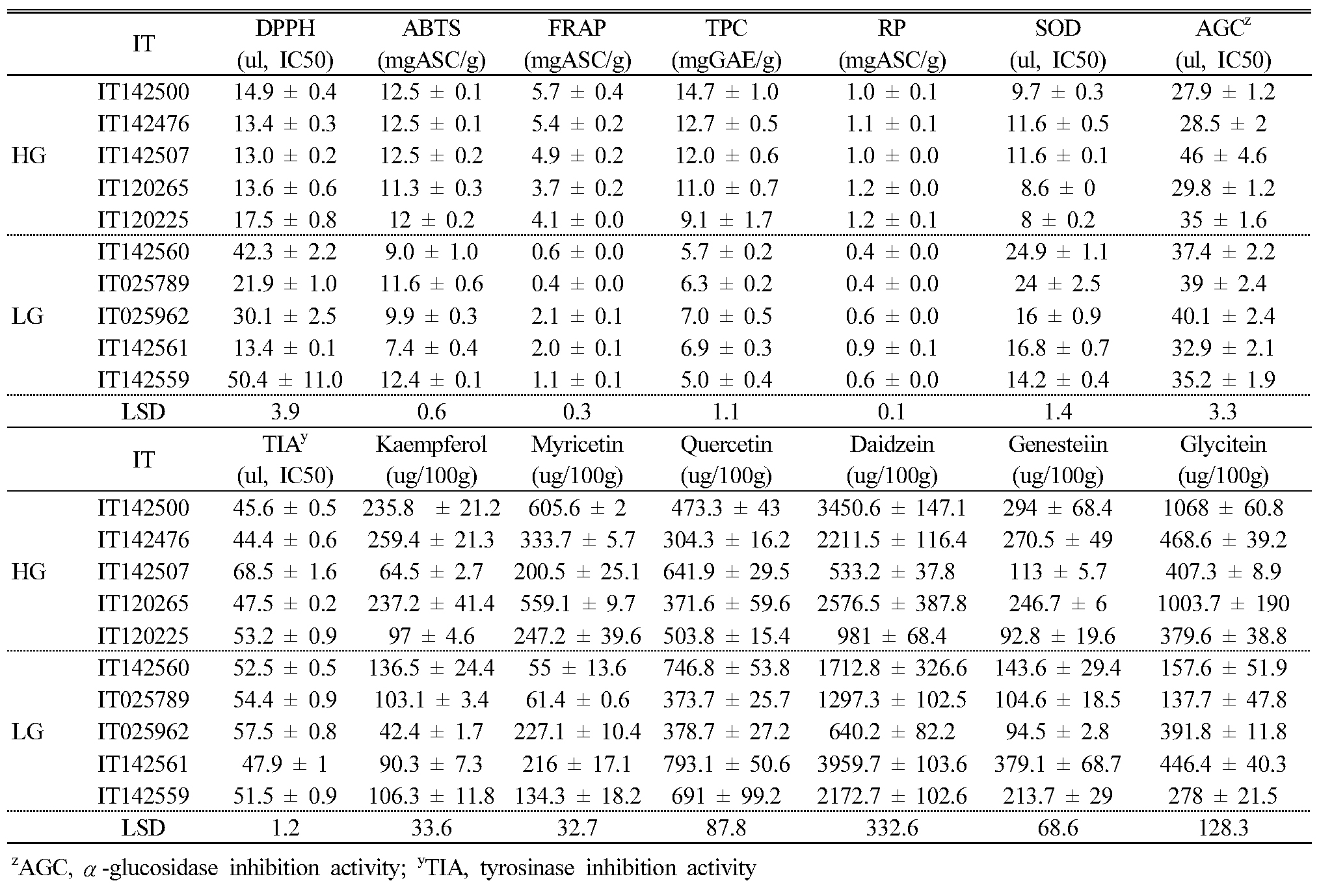
In order to confirm the relation among antioxidant activity, phytochemical contents, α-glucosidase inhibition activity, and tyrosinase inhibition activity, we selected 10 KABLs with high or low antioxidant activity based on the RACI results (Appendix 2), and we present the distribution of antioxidant activity in the leaf extracts from the 10 KABLs in Table 2. Among the five antioxidant activity assays, DPPH, FRAP, TPC, RP, and SOD were significantly higher in the high antioxidant group than in the low group, whereas ABTS showed no significant difference between the two.
Table 2. Descriptive statistics for antioxidant activity in the leaf extracts from 10 selected Korean adzuki bean landraces according to RACI |
|
Comparison of flavonoid content between the high and low antioxidant groups
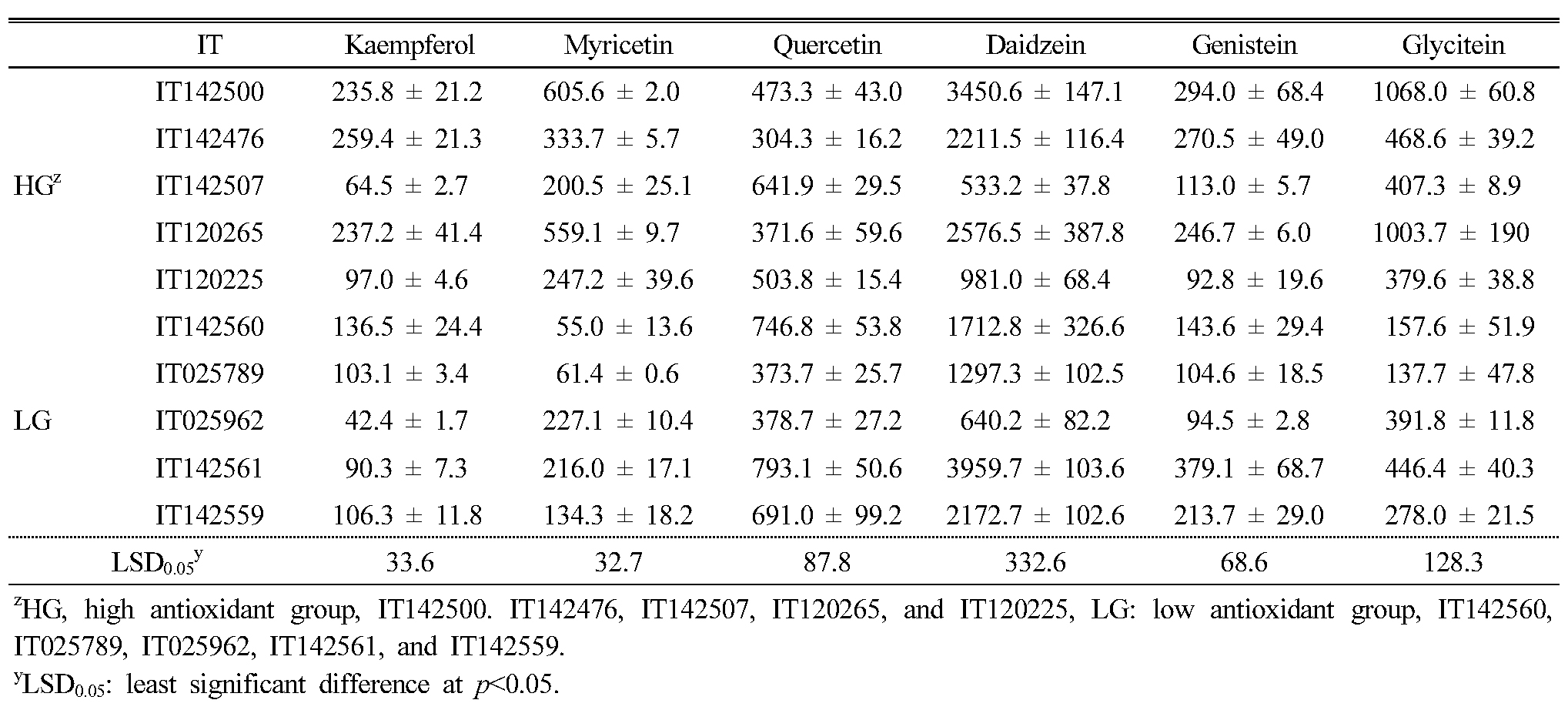
In order to compare the contents of phytochemicals between the high and low antioxidant groups, we measured six flavonoids (kaempferol, quercetin, myricetin, daidzein, glycitein, and genistein) related to antioxidant activity in 10 the selected landraces using HPLC. Among the six detected flavonoids, daidzein (36.1–67.3%, average of 51.9% in detected flavonoids) was the most abundant (Table 3). The daidzein, genistein, and glycitein content in the 10 selected KABLs ranged from 533.2 (IT142507, high group) to 3959 (IT142561, low group), 92.8 (IT120225, high) to 379.1 (IT142561, low), and 137.7 (IT025789, low) to 1068 (IT142500, high) ㎍/100g dried sample, respectively. Among the flavonols, kaempferol, myricetin, and quercetin content ranged from 42.4 (IT025962, low) to 259.4 (IT142476, high), 55.0 (IT142560, low) to 605.6 (IT142500, high), and 304.3 (IT142476, high) to 793.1 (IT142561, low) ㎍/100g dried sample, respectively.
Table 3. The flavonoid contents in the leaf extracts of 10 selected Korean adzuki bean landraces (㎍/100g) |
|
Comparison of α-glucosidase and tyrosinase inhibition activity between the high and low antioxidant groups
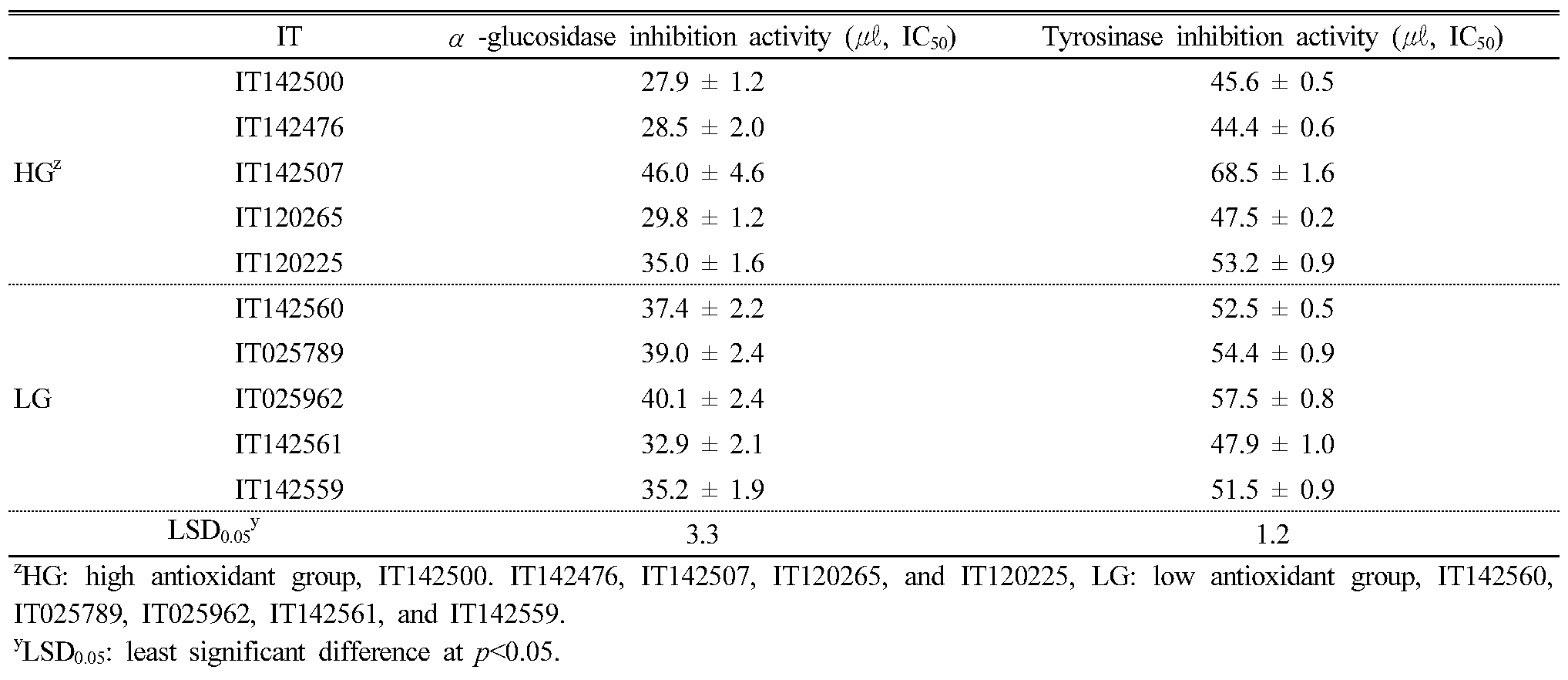
We tested the leaf extracts from the 10 KABLs we selected for their α-glucosidase and tyrosinase inhibition activity (Table 4); among the 10, IT142507 showed the lowest inhibition activity for both enzymes. AGC and TIA were higher in the high antioxidant group than in the low group except for IT142507 and IT 120225. AGC in the high group ranged from 0.34 to 0.55 (IC50, ㎍/㎖) and from 0.40 to 0.48 (IC50, ㎍/㎖) in the low group. TIA in the high and low groups ranged from 0.53 to 0.83 and 0.58 to 0.69 (IC50, ㎍/㎖), respectively.
Table 4. α-glucosidase inhibition and tyrosinase inhibition activity in the leaf extracts of 10 selected Korean adzuki bean landraces |
|
Correlations among antioxidant activity, phytochemicals, and α-glucosidase and tyrosinase inhibition activity in adzuki bean extracts
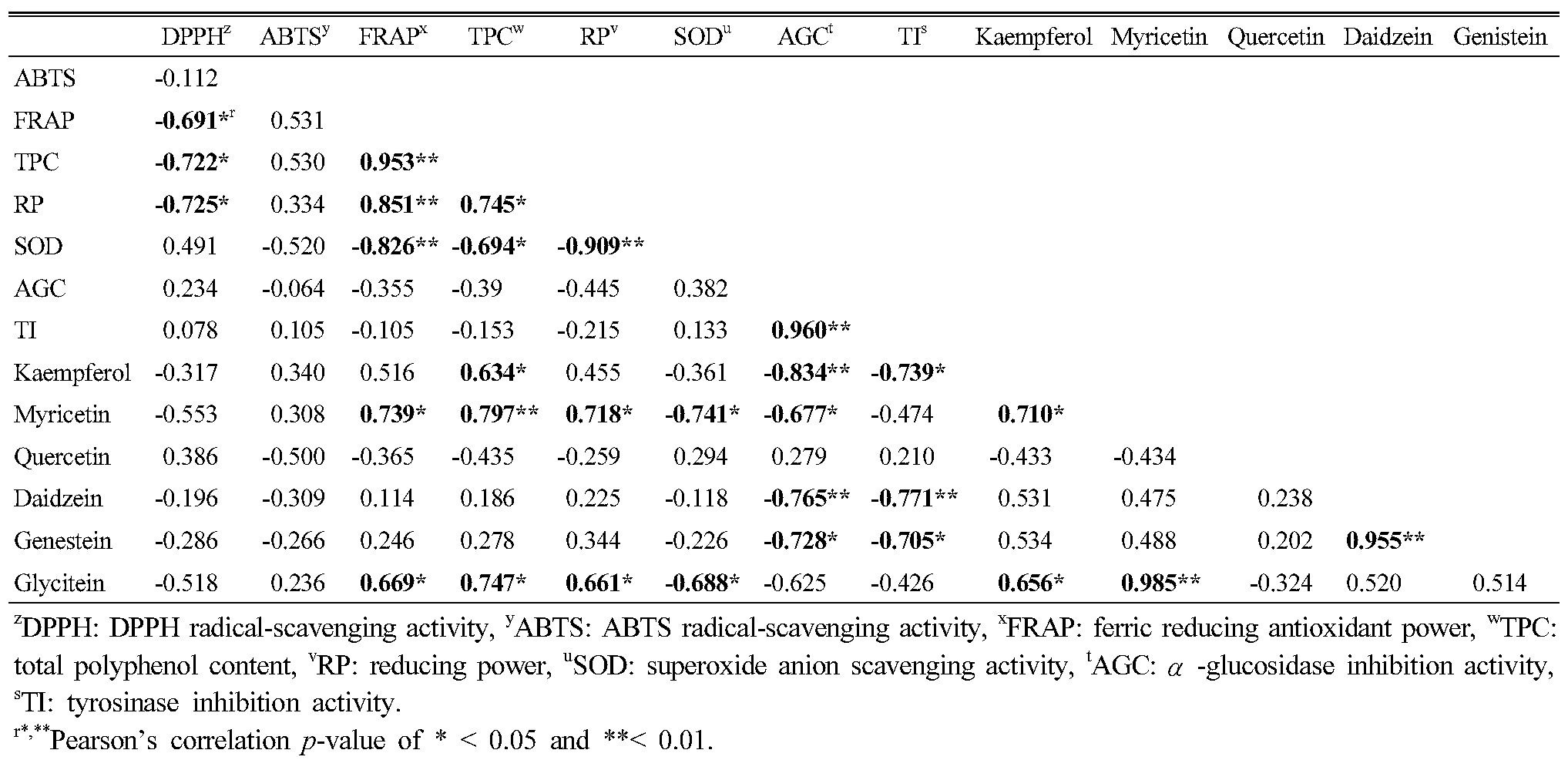
Table 5 shows the correlations among antioxidant activity, phytochemicals, α-glucosidase and tyrosinase inhibition activity; among them, ABTS showed no correlations with other factors. For five antioxidants, DPPH, FRAP, TPC, RP, and SOD, we detected positive or negative correlations among them, but there were no significant correlations for AGC or TIA activity. AGC and TIA did show a high positive correlation between the two (r=0.960, p<0.01). Among six flavonoids, kaempferol, myricetin and glycitein showed correlations with antioxidant activity. We detected negative correlations for AGC and TIA with kaempferol, daidzein, and genistein, and myricetin showed a negative correlation with AGC only.
Table 5. Correlations among antioxidants, α-glucosidase inhibition activity, tyrosinase inhibition activity, and phytochemical contents |
|
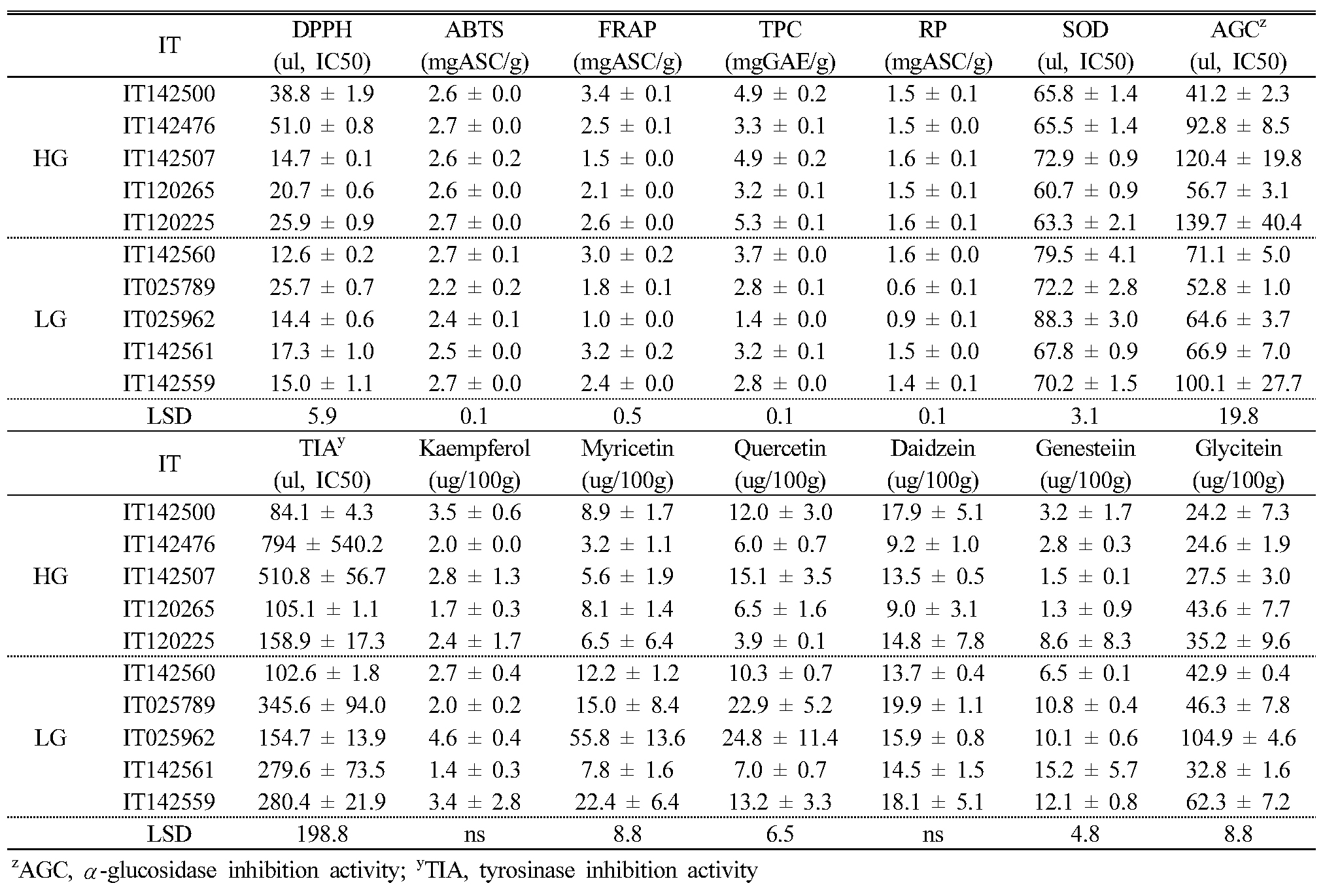
Comparison of antioxidant activity, phytochemicals, and α-glucosidase and tyrosinase inhibition activity between leaf and seed extracts
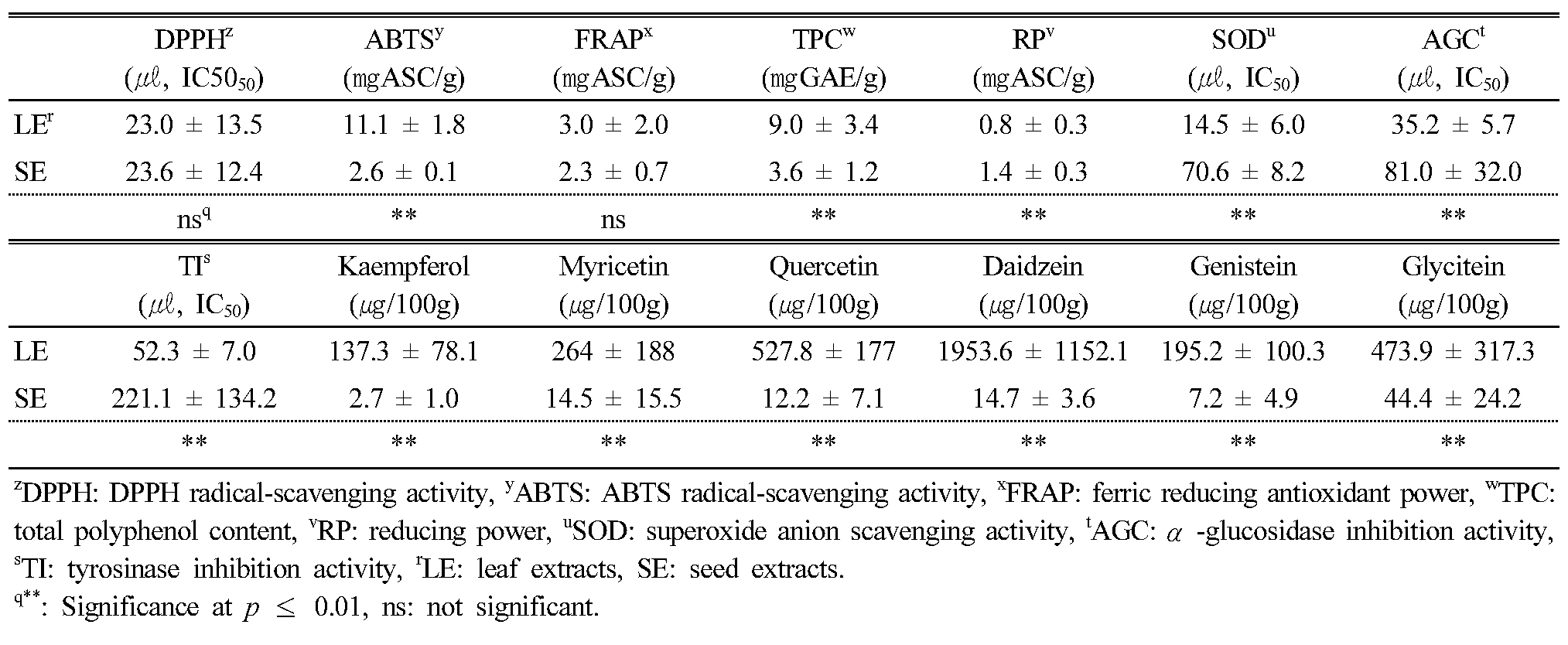
To confirm the potential uses for adzuki bean leaves, we compared leaf (LEs) and seed (SEs) extracts from the 10 selected KABLs based on phytochemical content and antioxidant and α-glucosidase and tyrosinase inhibition activity (Appendix 2 and 3). Among them, DPPH and FRAP showed no significant differences between the LEs and SEs (Table 6). For antioxidant activity, there was only more RP in the SEs (1.4 ± 0.3 ㎎ASC/g) than in the LEs (0.8 ± 0.3 ㎎ASC/g). There were, respectively, 2.3 and 4.2 times more AGC and TIA in the LEs (35.2 ± 5.7 and 52.3 ± 7.0 (㎕, IC50), respectively) than in the SEs (81.0 ± 32.0 and 221.1 ± 134.2 (㎕, IC50), respectively). For the six detected flavonoids, there was 133 times more daidzein in the LEs than in the SEs.
Discussion
For centuries, farming communities have continuously contributed to the evolution, enrichment, and maintenance of landrace diversity on farms (Brush, 1995; Jarvis et al., 2008). However, little has been done to understand the landrace diversity or to improve these landraces (Sthapit and Rao, 2009). suggested that landraces can be effectively improved by simple trait selection if they offer sufficient natural variations in their populations. In this study, we evaluated the antioxidant activity, phytochemical content, and α-glucosidase and tyrosinase inhibition activity in the leaf extracts of Korean adzuki bean landraces to identify their potential as new industrial resources. Our results revealed that the leaf extracts of these KABLs had different physiological activities and phytochemical contents. The success of a breeding program depends on the existence of genetic variability available to breeders (Hoisington et al., 1999). Landraces contain important genetic variability, which determines their ability to adapt to changes in their environments (Frankel et al., 1995). In addition, landraces provide useful variability for breeding provided that they are accompanied by characterization and agronomic evaluation (Allard, 1996; Frankel et al., 1995). This information is essential for the correct conservation of genetic variability and for the accessions to be of use in breeding programs (Vilaro et al., 2004). Our results could contribute to more efficient conservation and utilization of adzuki bean landraces.
In this study, the leaf extracts from the adzuki bean landraces had more efficient antioxidant activity than did the seed extract. Other studies reported that flavonoids are present in leaf extracts but absent in seed extracts (Archana et al., 2012; Nanna et al., 2013) ; their authors’ suggested that antioxidant, antimicrobial, and anti-inflammatory plant activity may be due to the presence of flavonoids. Flavonoids and total polyphenols are important secondary plant metabolites present at high levels in plants under stress (Koh et al., 2009; Stanojevic et al., 2009) because they play roles in reducing the oxidative stress caused by ROS (Patil and Jadhav, 2013). Flavonoids are free radical scavengers that prevent oxidative cell damage (Salah et al., 1995). Although our result did not show significant correlations between antioxidant activity and flavonoid content, we suggest that the higher flavonoid content in the leaf than the seed extracts might be responsible for the leaf extracts’ high antioxidant activity.
Plant antioxidants have been purported to have anti-aging properties and may prevent numerous diseases such as cancer, diabetes, and neurodegenerative diseases (Bansal et al., 2013). In our study, we measured the antioxidant activity of 223 Korean adzuki bean landraces using various methods. Additionally, based on the antioxidant activity findings, we evaluated the α-glucosidase and tyrosinase inhibitor activity in the leaf and seed extracts of the landraces. We found more α-glucosidase and tyrosinase inhibition activity in the leaf extracts, by 2.3 and 4.2 times, respectively, than in the seed extracts, although there were no significant correlations between antioxidant activity and α-glucosidase and tyrosinase inhibitor activity. Shoots and leaves have been reported to have higher phenolic content than that in other plant parts (Bernardi et al., 2008), and (Shaik et al., 2011) reported that leaf extracts of Lessertia frulescens showed more phenolics, flavonoids, alkaloids, and saponins than did seed extracts. In particular, (Oleszek and Stochmal, 2002) reported that flavonoid content in the seeds was characteristically low. Our results agreed with the previous studies: The phytochemical content in the leaf extracts was greater than in the seed extracts, resulting in more antioxidant and α-glucosidase and tyrosinase inhibitor activity.
α-Glucosidase inhibitors are potential agents for diabetes therapy given that glucosidases are involved in several important and relevant biological processes (Fontana Pereira et al., 2011). Our results showed that the flavonoid content in the leaf extracts was higher than in the seed extracts. In addition, among six flavonoids, kaempferol, myricetin, daidzein, and genistein had negative correlations with α-glucosidase inhibition activity (IC50). Flavonoids are naturally occurring phenolic compounds that are widely distributed in plants, and some of them have been described as glucosidase inhibitors (Cazarolli et al., 2009). Tadera et al. (2006) reported that anthocyanidin and isoflavone and flavonol groups were potent α-glucosidase inhibitors and cyanidin, myricetin, quercetin, genistein, kaempferol, and daidzein especially showed higher α-glucosidase inhibition activity. Other studies reported that some flavonoids such as quercetin, kaempferol, and daidzein are strong glucosidase inhibitors (Fontana Pereira et al., 2011; Kim et al., 2000). (Andrade-Cetto et al. (2008) reported that high flavonoid content in some plant extracts efficiently inhibits α-glucosidase activity.
In our study, we analyzed the tyrosinase inhibition activity of leaf and seed extracts from the adzuki bean, and we found higher tyrosinase inhibition, which could have been because of the higher flavonoid content in the leaf extracts than in those from the seeds (Matsuda et al., 1996). reported that several flavonoid derivatives including quercetin, myricetin, and myricetin-glucoside had varying degrees of inhibitory activity toward tyrosinase. Many flavonols and flavones such as quercetin, kaempferol, galangin, luteolin, chrysin, baicalein, and luteolin-7-O-glucoside were reported to have weak tyrosinase inhibitory activity (Kubo et al., 2000). In addition, Chang et al. (2007) reported that five isolated isoflavones such as daidzein, glycitein, and genistein showed tyrosinase inhibitory activity.
In this study, we evaluated the phytochemical content and in vitro antioxidant, α-glucosidase inhibition, and tyrosinase inhibition activity in the leaf extracts of Korean adzuki bean landraces using various methods for the first time. KABLs contain high phytochemicals and exhibited strong bioactivity. With the present study, we demonstrated that leaf extracts of adzuki beans are a new source of natural antioxidants, cosmetics, and medicines that could be used in industrial applications, which will strongly increase the utilization of adzuki bean by-products. This study laid a good foundation for developing and utilizing adzuki bean by-products as a new source.