Introduction
Materials and Methods
Sample Preparation and HPLC Analysis
Elastase Inhibition Assay
Cell Culture and Cell Viability Assay
UVB-Irradiated Cell Viability Assay
Mice UVB-Induced Models
Measurement of Skin Thickness, Erythema Level, and Transepidermal Water Loss (TEWL)
RNA Isolation and Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Western Blot Analysis
Statistical Analysis
Results
HPLC Profile of cG&Re
Effect of cG&Re on Elastase Inhibitios
Effect of cG&Re on Cell Viability
Effects of cG&Re on UVB-induced Mice Skin
Effect of cG&Re on Histochemical Changes
Effects of cG&Re on mRNA and Protein Expression of Skin Aging-Related Biomarkers
Protective Effects of Orally Administered cG&Re on Skin Tissues
Discussion
Introduction
Aging is defined as a gradual destruction of cells and tissues leading to organ damage over time (Choi et al., 2016). The skin, which is the largest organ, essentially forms a protective barrier against external factors such as pathogens and environmental factors. It visibly reflects the consequences of human aging (Liu et al., 2018a). Similar to other organs, the skin also undergoes changes over time due to the harmful effects of various factors such as hormonal and dietary variations (Rittie and Fisher, 2015). Extrinsic aging is premature aging due to the effects of extrinsic factors such as continuous exposure to ultraviolet (UV) radiation, environment pollution, and nicotine among others (McCullough et al., 2006). Among the numerous factors responsible for skin aging, ultraviolet (UV) radiation is considered to be the major external agent which causes skin aging. UV is classified into 3 categories: UVA (315-400 ㎚), UVB (280-315 ㎚) and UVC (100-280 ㎚) (Rosenthal et al., 1986). Chronic exposure to UV irradiation, especially that of UVA and UVB, produces an aging phenotype termed photoaging (Wen et al., 2018). Exposure of the skin to UVB, in particular, may lead to mitochondrial malfunction, oxidative stress, inflammation, and apoptosis which ultimately induces skin aging (Vogt et al., 1997). Furthermore, UVB irradiation also causes damage to skin cells by forming reactive oxygen species (ROS), which induce oxidative stress, an important mediator of cellular damage, including damage to lipids, membranes, proteins and DNA (Kim et al., 2007). Lesions caused by photo-aging lead to various pathological symptoms such as skin thickening, wrinkling, erythema, and dryness(Rittie and Fisher, 2002). In addition, an alteration of, or a reduction in, collagen and elastin, which accompany aging, makes skin fragile and lose its youthful appearance (Varani et al., 2000). ROS production activates the mitogen-activated protein kinase (MAPK) signaling pathway (Son et al., 2013). Cumulative evidence indicates that mitogen-activated protein kinases (MAPKs), such as extracellular-regulated protein kinase (ERK), Jun N-terminal kinase (JNK), and p-38, are closely associated with skin aging (Sun et al., 2015). Stimulation of MAPKs by UV irradiation may modulate cell proliferation, differentiation, inflammation, and apoptosis (Sun et al., 2015). Furthermore, MAPK activation also regulates the activator protein 1 (AP-1), which stimulates matrix metalloproteinase (MMP) expression in various cultured cells and animal tissues (Liu et al., 2018). Among MMPs, MMP-1 is a major collagenase which degrades type 1 collagen contained in skin connective tissues (Rittie and Fisher, 2002). Thus, activation of MMP-1 by UV-irradiation and subsequent degradation of collagen are viewed as hallmarks of skin inflammation as well as photo-damage (Inomata et al., 2003). Recently, there have been many reports on the functional effects of plant extracts (Koo et al., 2018; Sekhon-Loodu et al., 2019). Natural plant substances including polyphenols and flavonoids with anti-oxidant effects are being discovered and studied to alleviate these symptoms of aging (Kaurinovic and Vastag, 2019). Grapefruit trees, similar to other citrus trees, are perenifolium trees. Grapefruit contains an abundance of ascorbic acid and polyphenolics and exerts diversified pharmacological effects including those which are antigenotoxic and chemopreventive (Cristobal-Luna et al., 2018; Garcia-Martinez et al., 2018). Rosemary (Rosmariunus officinalis L.), found mainly in the Mediterranean region, is a fragrant plant from the Lamiaceae family. Mounting evidence indicates that rosemary exhibits pharmacological potential by exerting antioxidant, anticancer and anti-diabetic effects which are mainly associated with the presence of a high degree of phenolic constituents (Cheung and Tai, 2007; Cuvelier et al., 1996; Erenmemisoglu et al., 1997; Xie et al., 2006). Therefore, it is assumed that the mixture of both plant extracts could exposed synergic effect for improving anti- aging symptoms in skin. Numerous studies have suggested that a combination of natural plant extract has been shown to more improve the beneficial effects than individual single extract or compound (Zhou et al., 2016; Chanda and Rakhollya, 2011). Perez-Sanchez et al. reported the protective effects of grapefruit and rosemary extracts on UV-induced damage in skin cell models and humans. They revealed that oral administration of grapefruit and rosemary extracts (250 ㎎) in humans resulted in a significant minimal erythema dose (MED) increase after 8 weeks (34%, p < 0.05), although the precise molecular mechanisms underlying the effect of these extracts were not elucidated.
The protective effects of orally as well as topically administered grapefruit and rosemary extract combination on UVB-induced skin aging and mechanisms underlying such effects have not been extensively studied. In the current study, we explored the anti-skin aging effects of a combination of grapefruit and rosemary extracts (cGℜ 1:1 ratio) on UVB-stimulated models and the molecular mechanisms underlying these effects.
Materials and Methods
Sample Preparation and HPLC Analysis
The herbal extract was a combination of grapefruit and rosemary extracts supplied by Monteloeder S.L. (Miguel Servet 16, Elche, Alicante, Spain), obtained from dried rosemary (Rosmarinus officinalis) leaves and grapefruits (Citrus paradisi) at a 1:1 ratio. The extract was standardized with naringin and carnosic acid as reference compounds for grapefruit and rosemary, respectively. The naringin content was 18.0±1.0 g/100 g on a dry weight basis, while the carnosic acid content was 2.0±0.2 g/100 g on a dry weight basis.
For HPLC analysis, a combination of grapefruit and rosemary (2 g; 1:1 ratio) was extracted using 50 ml of dimethyl sulfoxide (DMSO). The sample was filtered into HPLC via a 0.45-㎛ syringe filter. HPLC was performed using an Agilent 1,200 system equipped with a model G1312A binary LC pump, an auto sampler, and a diode-array detector. A C-18 (LiChrospher 100 RP-18) column (250 ㎜ x 4.0 ㎜ id and 5 ㎛ particle size) was used to separate all polyphenols and flavanones from the extract. The standards were purchased from Sigma Chemicals (St. Louis., MO, USA). Peaks were detected at 235 ㎚. Chromatographic separations were performed via a gradient mobile phase consisting of 2.5% acetic acid prepared in nanopure water (a) and 100% acetonitrile (b) with the following solvent gradient: 0 min, 95% A; 15 min, 80% A; 25 min, 75% A; 40 min, 50% A; 70 min, 20% A; 80 min, 95% A. The injection volume was 20 μL, mobile phase flow rate was 1 mL/min, oven temperature was 30℃ and the detection wavelength was 235 ㎚
Elastase Inhibition Assay
Elastase inhibition activity of cG&Re was analyzed according to a previously reported method with a slight modification (Kraunsoe et al., 1996). In brief, 0.1 M Tris-HCl (pH 8.0) containing 0.78 mM N-succinyl-Ala-Ala-Ala-p-nitroanilide (S4760, Sigma-Aldrich, St. Louis, MO, USA) was added together with 0.04 unit/mL elastase (45124, Sigma-Aldrich). Two microliters each of various concentrations of cG&Re and 100 μL of substrate solution were mixed following the addition of elastase enzyme (100 μL) in a 96-well plate (SPL Life Science Co., Ltd., Pocheon, Korea). Enzyme and substrate reaction was monitored 25 times at 1-min intervals, using a UV spectrophotometer (VICTOR3, Perkin Elmer, Wellesley, MA) at 405 ㎚ and 37℃. Epigallocatechin gallate (EGCG) served as a positive control.
Cell Culture and Cell Viability Assay
HaCaT immortalized human keratinocytes were obtained from AddexBio Technologies (T002001, San Diego, CA, USA). The cells were cultivated in DMEM, supplemented with 10% fetal bovine serum (FBS) and 1% penicillin- streptomycin (P/S) at 37℃ in a 5% CO2 atmosphere. MTT assay was conducted using 3-(4,5-dimethyl-2-thiazolyl)-2,5- diphenyl-2H-tetrazolium bromide (MTT) in order to evaluate cell viability. Briefly, HaCaT cells (1 x 105 cells/mL) were placed in 96-well plates. Subsequent to HaCaT cell growth reaching 80-90 % confluency, the cells were treated with 1, 3, 10, 30, and 100 ㎍/mL of cG&Re for 24 h in a CO2incubator at 37℃. The media was changed to MTT (5 ㎎/mL in PBS) solution following incubation for an additional 1 h. Next, MTT solution was removed and 100 μL of DMSO was added to dissolve formazan in each well. The formation of insoluble formazan was measured using a microplate reader at 595 ㎚ (Victor3, Perkin Elmer, MA, USA).
UVB-Irradiated Cell Viability Assay
HaCaT cells were seeded on 96-well plates (1 x 105 cells/ mL), and treated with different doses of cG&Re for 24 h. The media was changed to 1X PBS (100 μL) and the plates were exposed to 30 mJ/㎠ of UVB using a UV lamp (Bio-Link crosslinker). Next, media were removed and predetermined concentrations of cG&Re were applied in fresh media. After 24 h exposure to UVB, 100 μL of MTT solution was added to the cells following 1 h incubation. The media was removed and insoluble formazan crystals formed in intact cells were solubilized in 100 μL of DMSO. Absorbance was measured using a microplate reader (Victor3, Perkin Elmer, MA, USA).
Mice UVB-Induced Models
Male BALB/c mice, aged 7 weeks and weighting 18-22 g were obtained from Samtako, Korea. The mice were maintained at 22 ± 1℃ and a humidity of 55 ± 1% under 12 h dark/light cycles. They were fed a commercial diet and had ad libitum access to water. The study was approved by the Laboratory Animal Ethics Committee (KNU 2018-0073), Kyungpook National University (Daegu, Korea). For topical application of a cG&Re studies as follows, the mice were divided into 5 groups, each group consisting of 5 mice intended for topical application; group I: Vehicle treated, group II: UVB-irradiated only; group III: UVB-irradiation with epigallocatechin gallate (EGCG) (10 ㎎/mL), group IV: UVB-irradiation with cG&Re (10 ㎎/mL), group V: UVB- irradiation with cG&Re (50 ㎎/mL). A 150 μL sample was applied to the dorsal skin of each mouse followed by exposure to UVB, which was gradually increased from 1 MED to 4 MED. 1, 3-butylene glycol with saline (3:7, v/v) was used as the vehicle, and EGCG was used as the positive control. For purposes of oral administration, mice were treated with cG&Re dissolved in deionized water at a concentration of 50 ㎎/day/kg (p.o.) followed by UVB exposure according to the above protocol.
Measurement of Skin Thickness, Erythema Level, and Transepidermal Water Loss (TEWL)
Skin thickness was measured using a digimatic thickness gauge (Code No. 547-315, Mitutoyo, Kanagawa, Japan). A colorimeter (CR-400, Minolta, Tokyo, Japan) and a vapometer (Delfin Technologies, Stamford, Connecticut) were used to assess the level of erythema and transepidermal water loss (TEWL) in dorsal skin, respectively, based on the manufacturers’ instructions as described elsewhere.
Histochemical Analysis
After the mice were sacrificed, dorsal skin tissue samples were obtained. Dorsal skin tissues were immobilized in 10% formaldehyde solution in phosphate buffered saline (PBS) for 24 h and embedded in paraffin. The slices were cut into 5-㎛ thick sections, which were deparaffinized prior to being soaked in acetone and washed in PBS. Slides were treated with 3 % hydrogen peroxide in methanol due to peroxidase activity and epitope retrieval was conducted. Subsequently, samples were treated with 10 % normal goat serum for 1 h. SIRT1 (ab166821, Abcam), MMP-1 (ab137332, Abcam), and IL-1β (ab9722, Abcam) primary antibodies were incubated with the sections overnight. Hematoxylin and eosin (H&E) staining and Masson’s trichrome staining was conducted to evaluate skin thickness and collagen content in the dermis, respectively. Stained slides were imaged using an ECLIPSE TE2000-U microscope (Nikon, Japan).
RNA Isolation and Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Total RNA from mice dorsal skin samples was isolated using TRIzol reagent (Life Technology, CA, USA) in accordance with the manufacturer’s protocol. Equal amounts of RNA (2 ㎍) was used as a template for cDNA synthesis using RT-&GO Master Mix (MP Biomedicals, CA, USA). The amplified products were electrophoresed on 1% agarose gel, visualized with ethidium bromide and detected using Image Lab software (ChemiDoc). GAPDH was used for normalization. The primer sets for RT-PCR were as follows: MMP-1, forward (F): 5’-GGC TCA TGA ACT GGG TCA CT-3’ and reverse (R): 5’-ATG TGG TGT TGT TGC ACC TG-3’; MMP-13, F: 5’-AGG CCT TCA GAA AAG CCT TC-3’ and R: 5’-CCC ACC ATA GTT TGG TCC AG-3’; SIRT1, F: 5’- GGT AGA GCC TGC ATA GAT CTT CA-3’ and R: 5’-TGG CAG TAA TGG TCC TAA CTG GG-3’.
Western Blot Analysis
Homogenized skin tissues were lysed in lysis buffer using protease and phosphatase inhibitor. The proteins were quantified using Bradford protein method (Kruger., 2009). Fifty ㎍ of proteins were separated via 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes (Whatman, Dassel, Germany). The membranes were blocked with 5% skim milk or BSA for 1 h and washed with Tris-buffered saline including Tween-20 (TBST) for 1 h at 15 min intervals. Next, the membranes were incubated with primary antibodies against MMP-1, SIRT1, ERK 1/2, phospho-ERK 1/2, phospho-JNK, p38, and phospho-p38 at 4℃ overnight. After rinsing, the membranes were incubated with anti-rabbit IgG-horseradish peroxidase (HRP) (BETHYL, USA), anti-mouse IgG-horseradish peroxidase (HRP) (BETHYL, USA) and anti-goat IgG-horseradish peroxidase (HRP) (BETHYL, USA) secondary antibodies for 2 h. Proteins were detected using an ECL solution system (ChemiDocTM XRS+, BIO-RAD).
Statistical Analysis
All data are expressed as mean ± standard deviation (SD). Statistical values were analyzed via a Student’s t-test and one-way analysis of variance (ANOVA) with Fisher’s Least Significant Difference (LSD) test using the statistical package for the social sciences (SPSS) software (SPSS Inc., Chicago, IL). Statistical significance was set at p < 0.05 and p < 0.01.
Results
HPLC Profile of cG&Re
The cG&Re was standardized with naringin and carnosic acid as reference compounds for grapefruit and rosemary, respectively. The naringin and carnosic acid contents on a dry weight basis were 18.0±2.0 g/100 g and 2.0±0.2 g/100 g, respectively. Five peaks were identified on the HPLC chromatogram as follows: naringin (21.7 min); rosmarinic acid (23.8 min); poncirin (32.6 min); carnosol (51.5 min), and carnosic acid (58.3 min); (Fig. 1A). The above 5 polyphenol compounds were identified by comparing each peak (peak 1-5) with a standard mixture using HPLC. These results were further utilized to elucidate the mechanism underlying cG&Re effects.
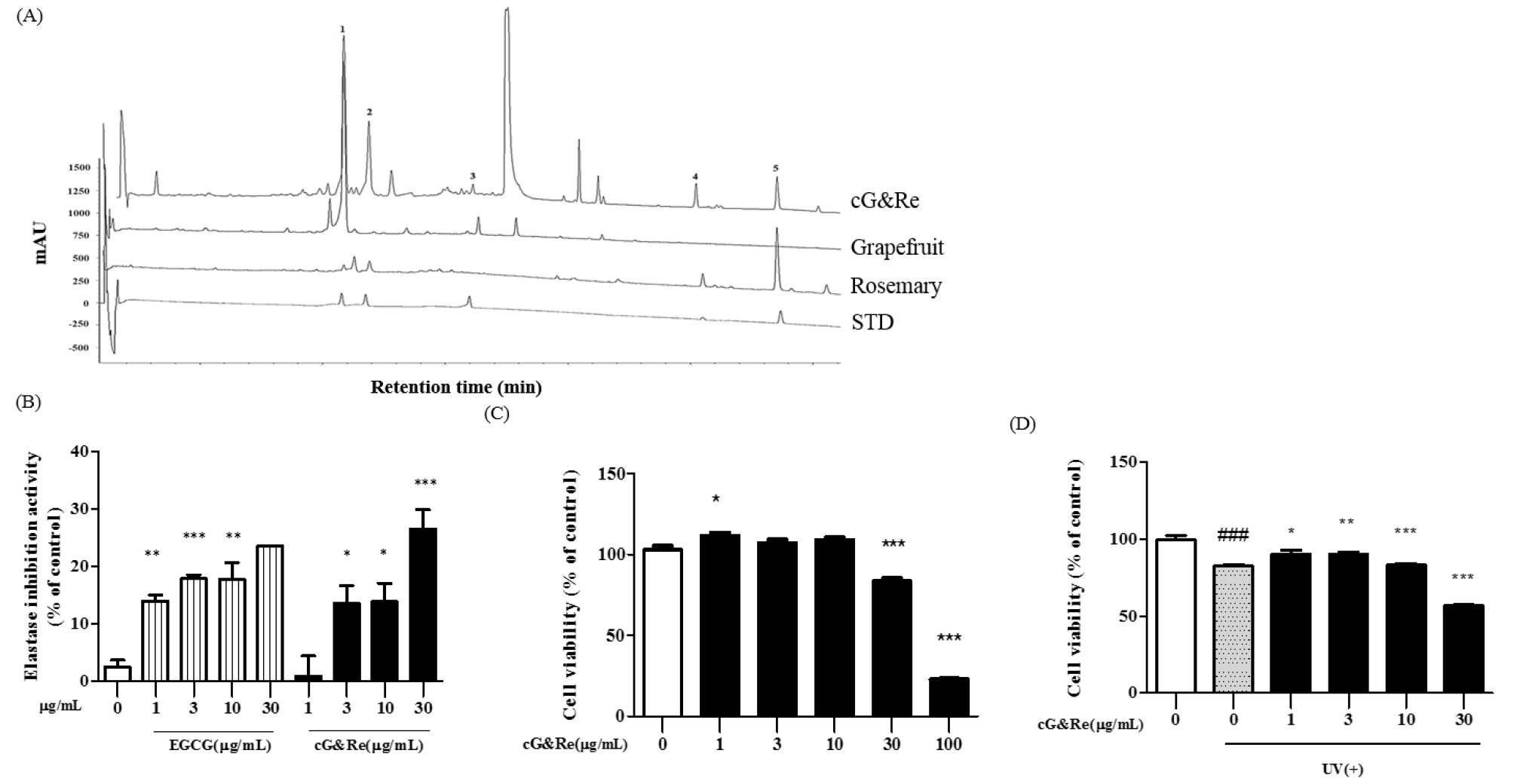
Fig. 1.
HPLC chromatogram, elastase inhibitory activity and cell vitality of cG&Re. (A) A classical feature of 5 standard compounds (4th lane), grapefruit extract (2nd lane), rosemary extract (3rd lane), and a combination of grapefruit and rosemary extract (1st lane, cG&Re). Peak 1, naringin; peak 2, rosmarinic acid; peak 3, poncirin; peak 4, carnosol; peak 5, carnosic acid. (B), Elastase inhibitory activity by cG&Re. Activity was assayed according to procedures described in the Materials and Methods section. EGCG was used as a positive control. (C-D), MTT assay was carried out to evaluate cell viability using HaCaT cells with (D) or without (C) exposure to UVB, with cG&Re treatment. ###= P < 0.001 and * = P < 0.05, ** = P < 0.01, *** = P < 0.001 compared with non-treated.
Effect of cG&Re on Elastase Inhibitios
Elastase is an enzyme which breaks down elastin and elastic fibers. Our result revealed that the cG&Re extract showed potential for significantly inhibiting elastase (Fig. 1B). Results indicated that cG&Re at 30 ㎍/mL exhibited higher elastase inhibition activity compared with the same concentration of EGCG, used as the positive control.
Effect of cG&Re on Cell Viability
Before initiating the cell experiment, a MTT assay was implemented to test the cytotoxicity of 1, 3, 10, 30, and 100 ㎍/mL of cG&Re. Up to 30 ㎍/mL of the cG&Re extract displayed no cytotoxicity in HaCaT cells (Fig. 1C). Based on these results, concentrations of 1, 3, 10 and 30 ㎍/mL of cG&Re were selected for use in further studies. UVB-induced cell toxicity was analyzed following exposure to UV irradiation (30 mJ/㎠) which causes cell death in HaCaT cells. Following 24 h of exposure to 30 mJ/㎠ of UVB, there was an approximately 20% decrease in cell viability. Interestingly, treatment with cG&Re extract at concentrations of 1, 3, and 10 ㎍/mL significantly protected cell viability by suppressing UVB-induced cell death. These results suggested that cG&Re treatment may protect cell viability from UV irradiation (Fig. 1D).
Effects of cG&Re on UVB-induced Mice Skin
UV treatment triggered wrinkles, roughness, dryness, flakes and redness of skin (2nd image; Fig. 2A) compared to that in the untreated group (1st image; Fig. 2A). Interestingly, topical application of cG&Re prevented UV-induced skin aging lesions (4th-5th images vs 3rd image of a positive control; Fig. 2A). The UVB-irradiated group dramatically increased skin thickness (2nd column; Fig. 2B) compared with the untreated group (1st column; Fig. 2B). In addition, cG&Re treatment significantly inhibited UVB-induced epidermal thickening. Moreover, UVB irradiation substantially induced skin erythema in mouse dorsal skin, wherein cG&Re treatment alleviated this (Fig. 2D). However, treatment with cG&Re strongly suppressed transepidermal water loss (TEWL) compared to that in the UVB-irradiated group (Fig. 2E). ###= P < 0.001 and * = P < 0.05, ** = P < 0.01, ***= P < 0.001 compared with non-treated.
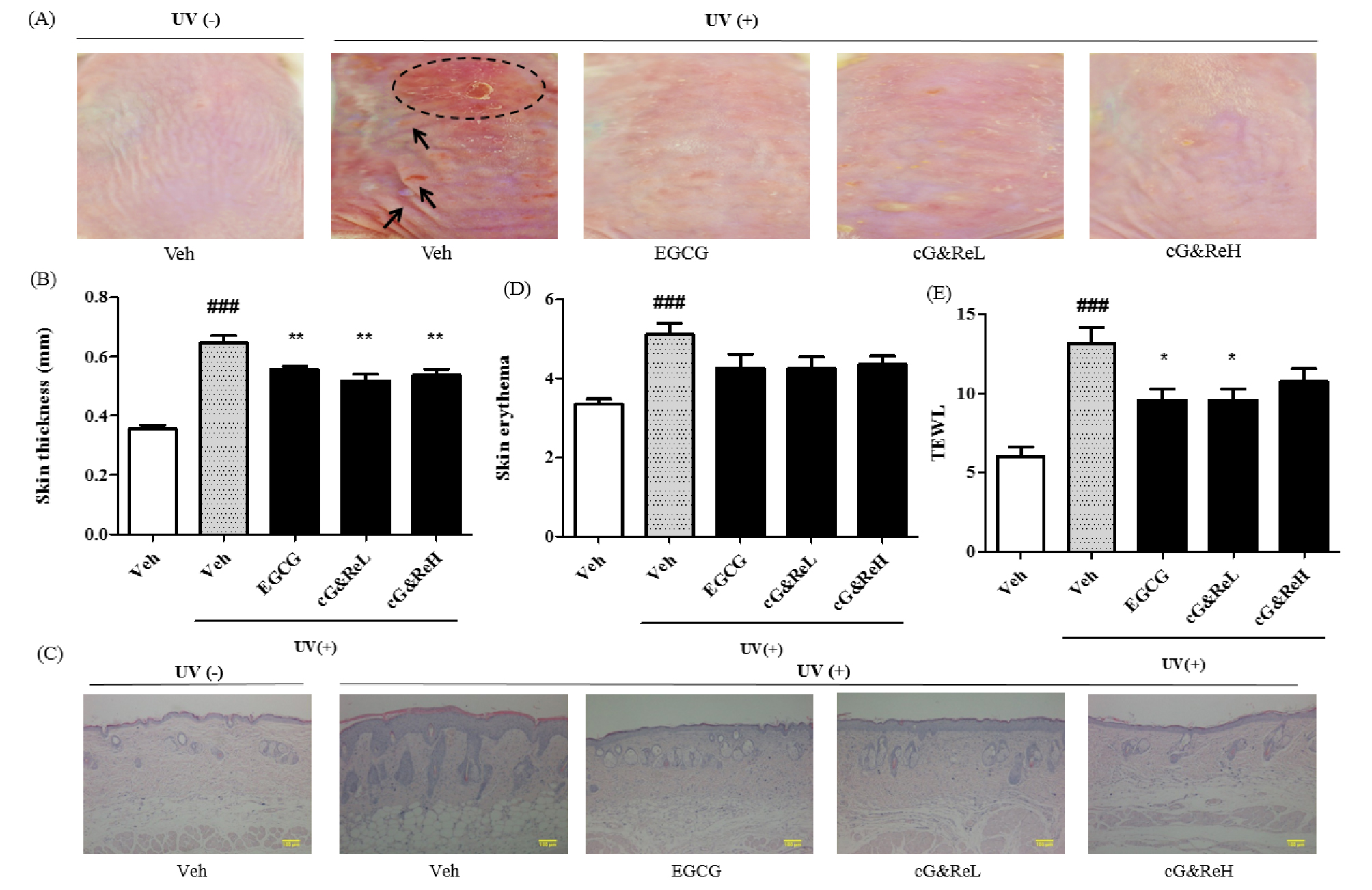
Fig. 2.
Preventive effects of cG&Re. (A), A classical feature of protective effects by CG&Re treatment was shown on the UVB-induced skin aging mouse model. Dotted circle denotes inflammatory erythema on the skin, while arrows indicate wrinkles. Veh, vehicle; UV(-), non UVB-irradiated; UV(+), UVB-irradiated. cG&ReL and cG&ReH mean the low (10 ㎎/mL) and high (50 ㎎/mL) doses of the applied extract on the skin. (B, D~E), Skin thickness, skin erythema and TEWL were calculated by digimatic thickness gauge (Code No. 547-315, Mitutoyo, Kanagawa, Japan), colorimeter (CR-400, Minolta, Tokyo, Japan), and vapometer (Delfin Technologies, Stamford, Connecticut) were used to assess erythema level and transepidermal water loss (TEWL) in dorsal skin, respectively, as shown in (E). (E), From the skin tissues of (A), all samples were sectioned at a 5-㎛ thickness and the sections were deparaffinized prior to being soaked in acetone and washed in PBS, as described in Materials and Methods section. Hematoxylin and eosin (H&E) staining was conducted and stained slides were photographed using a microscope (ECLIPSE TE2000-U, Nikon, Japan). ###= P < 0.001 and * = P < 0.05, ** = P < 0.01 compared with non-treated. Scale bar: 100 ㎜.
Effect of cG&Re on Histochemical Changes
Next, we evaluated collagenase inhibitory activity via Masson’s trichrome staining of dorsal skin tissues. Although the UVB-irradiated group showed low collagen density (2nd image; Fig. 3A), cG&Re treatment restored UVB-induced decrease in collagen contents in the dermis (4th-5th images vs 2nd -3rd images; Fig. 3A). An immunohistological analysis was performed to estimate the expression of specific proteins and cytokines in the skin tissues. Our results demonstrated that cG&Re treatment stimulated SIRT1 secretion (Fig. 3B) which was stained brown in the dermis. On the other hand, expression of MMP-1 and IL-1β (Figs. C and D), which was stained brown stains in the epidermis, decreased in the cG&Re-treated group (4th-5th images; Figs. 3C and D) compared to that in the UVB-irradiated group (2nd images; Figs. 3B-D). These data indicated that cG&Re treatment protected protein expression of skin ageing-related biomarkers by inhibiting MMP-1 and IL-1β and activating SIRT-1.
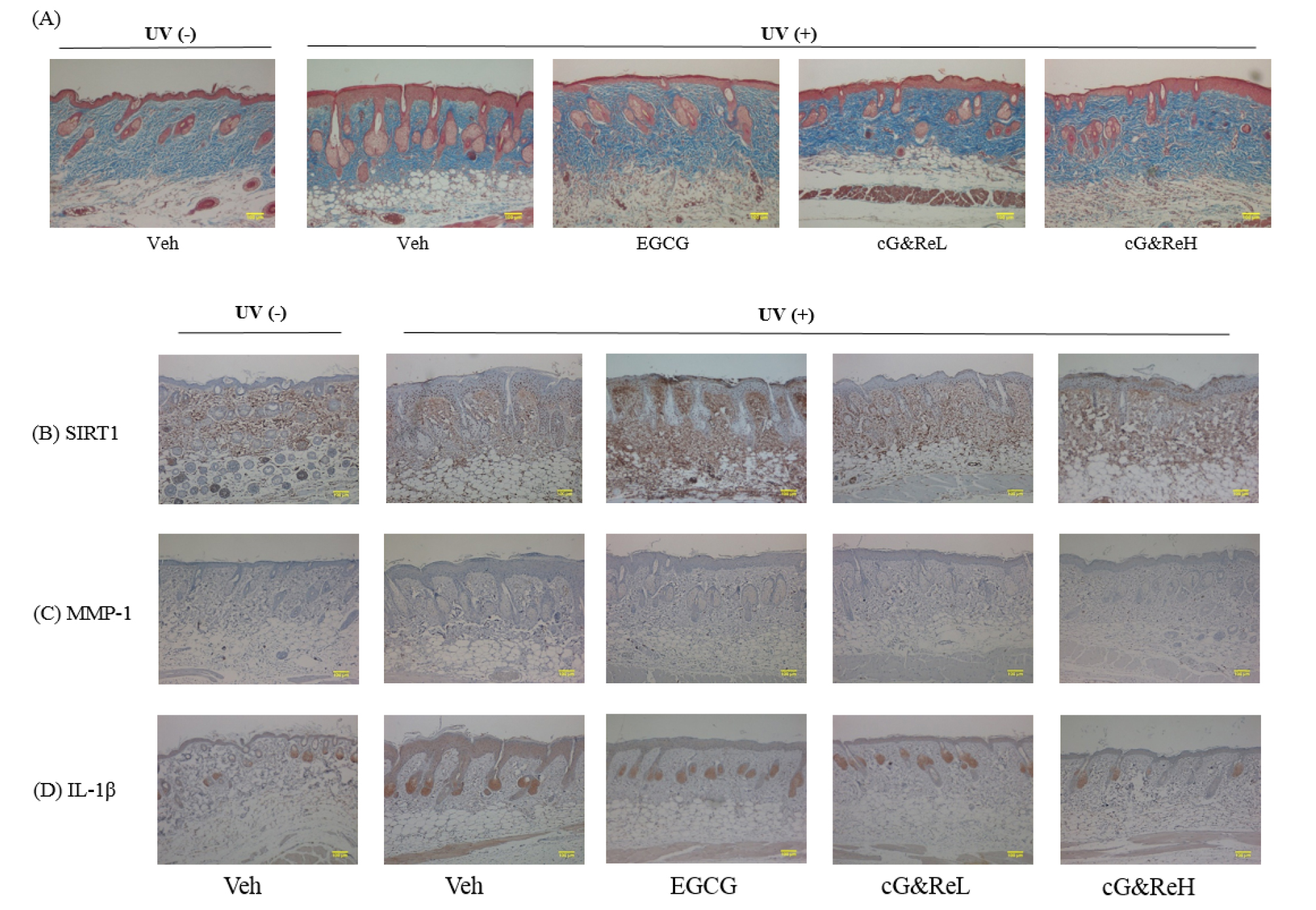
Fig. 3.
Effects of cG&Re on histochemical changes and skin aging-related biomarkers. (A), A characteristic image depicting protective effects of CG&Re treatment in the UVB-irradiated mouse skin aging model. Masson’s trichrome staining was conducted to inspect collagen content in dermis. Stained slides were illustrated using a microscope (ECLIPSE TE2000-U, Nikon, Japan). (B-D), SIRT1, MMP-1, and IL-1β were examined to assess the anti-skin aging activity in the animal model using hematoxylin and eosin (H&E) staining. Veh, vehicle; UV(-), non UVB-irradiated; UV(+), UVB-irradiated. cG&ReL and cG&ReH indicate low (10 ㎎/mL) and high (50 ㎎/mL) doses of the applied extract on the skin. Scale bar: 100 ㎜.
Effects of cG&Re on mRNA and Protein Expression of Skin Aging-Related Biomarkers
Expression of MMP-1, MMP-13, and SIRT 1 mRNA was examined using RT-PCR in the animal tissues (Fig. 4A). MMP-1 and MMP-13 mRNA was overexpressed due to UV-irradiation (2nd bands of 1st and 2nd images; Fig. 4A), whereas cG&Re treatment suppressed expression of MMP-1 and MMP-13 mRNA in a dose-dependent manner. Notably, expression of MMP-1 mRNA was significantly reduced by a high concentration (50 ㎎/mL) of cG&Re compared to that in the UVB-irradiated group (2nd band vs 3rd-5th bands). However, cG&Re did not affect SIRT 1 mRNA expression (1st-5th bands of 3rd image). Next, western blot analysis was conducted to investigate protein expression of MMP-1 and SIRT1. The data showed increased MMP-1 and SIRT 1 expression in the UVB-treated group (2nd band; Fig. 4B) compared to that in the non-treated group (1st band), but cG&Re treatment inhibited protein expression of MMP-1 (4th-5th bands). Similar to the pattern of RT-PCR results, cG&Re had no effect on SIRT 1 expression (2nd image; Fig. 4B). To elucidate the precise mechanism underlying the anti-skin aging effect of cG&Re, phosphorylation of mitogen-activated protein kinases (ERKs, JNK, and p38) was assessed. UVB upregulated phosphorylation of p38, but cG&Re inhibited UVB-mediated phosphorylation of p38 at high concentrations (1.7-fold) (Fig. 4C). In addition, phosphorylation of ERKs and JNK affect cG&Re treatment, suggesting that MAPK signaling was directly associated with the protection afforded by cG&Re activation.
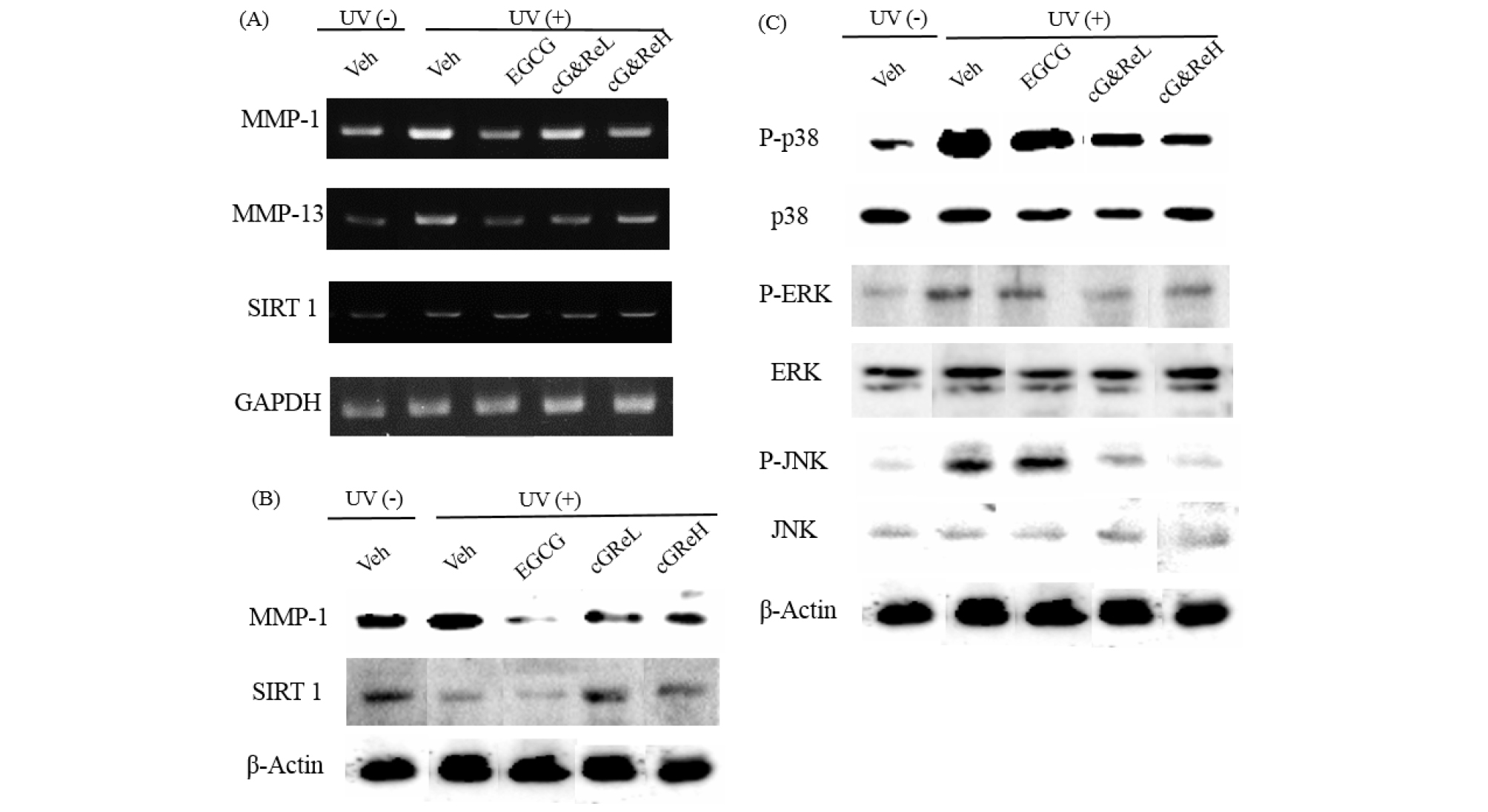
Fig. 4.
mRNA and protein expression of aging-related biomarkers and signaling molecules induced by cG&Re. (A), Samples of dorsal skin tissue were obtained from sacrificed mice. Total RNA of mice dorsal skin samples was isolated using TRIzol reagent (Life Technology, CA, USA) in accordance with the manufacturer’s protocol, followed by RT-PCR which was performed as described earlier. (A), Homogenized skin tissues were lysed in lysis buffer using protease and a phosphatase inhibitor. Separated proteins were transferred to nitrocellulose membranes (Whatman, Dassel, Germany) for the purpose of analyzing the expression of MMP-1 (B), SIRT1 (B), and MAPK cascades (p38, ERK and JNK) (C). Veh, vehicle; UV(-), non UVB-irradiated; UV(+), UVB-irradiated; cG&ReL and cG&ReH represent low (10 ㎎/mL) and high (50 ㎎/mL) dose of the extract, respectively.
Protective Effects of Orally Administered cG&Re on Skin Tissues
To further scrutinize the protective effect of orally administered cG&Re, we administered cG&Re at a concentration of 50 ㎎/kg and conducted an immunohistochemical analysis of SIRT-1, MMP-1 and IL-1β expression in UV-treated mouse tissues. Interestingly, image data indicated that the dermis of UV-untreated tissues had a thicker epidermal layer (1st image vs 2nd mage; Fig. 5A) and a thinner collagen layer (1st image vs 2nd image; Fig. 5B) than that of UV-treated tissues, whereas cG&Re treatment effectively reduced its inflammatory response in the tissues. In the same manner, there was no striking inflammatory response in the cG&Re-treated tissues (Fig. 5C). Immunohistochemical data clearly showed an increased level of SIRT-1, as well as decreased levels of MMP-1, and IL-1β (2nd image vs 3rd image for each protein; Fig. 5C). These data suggested that orally administered cG&Re affected expression of anti-inflammatory and skin ageing-related biomarkers in the skin tissues.
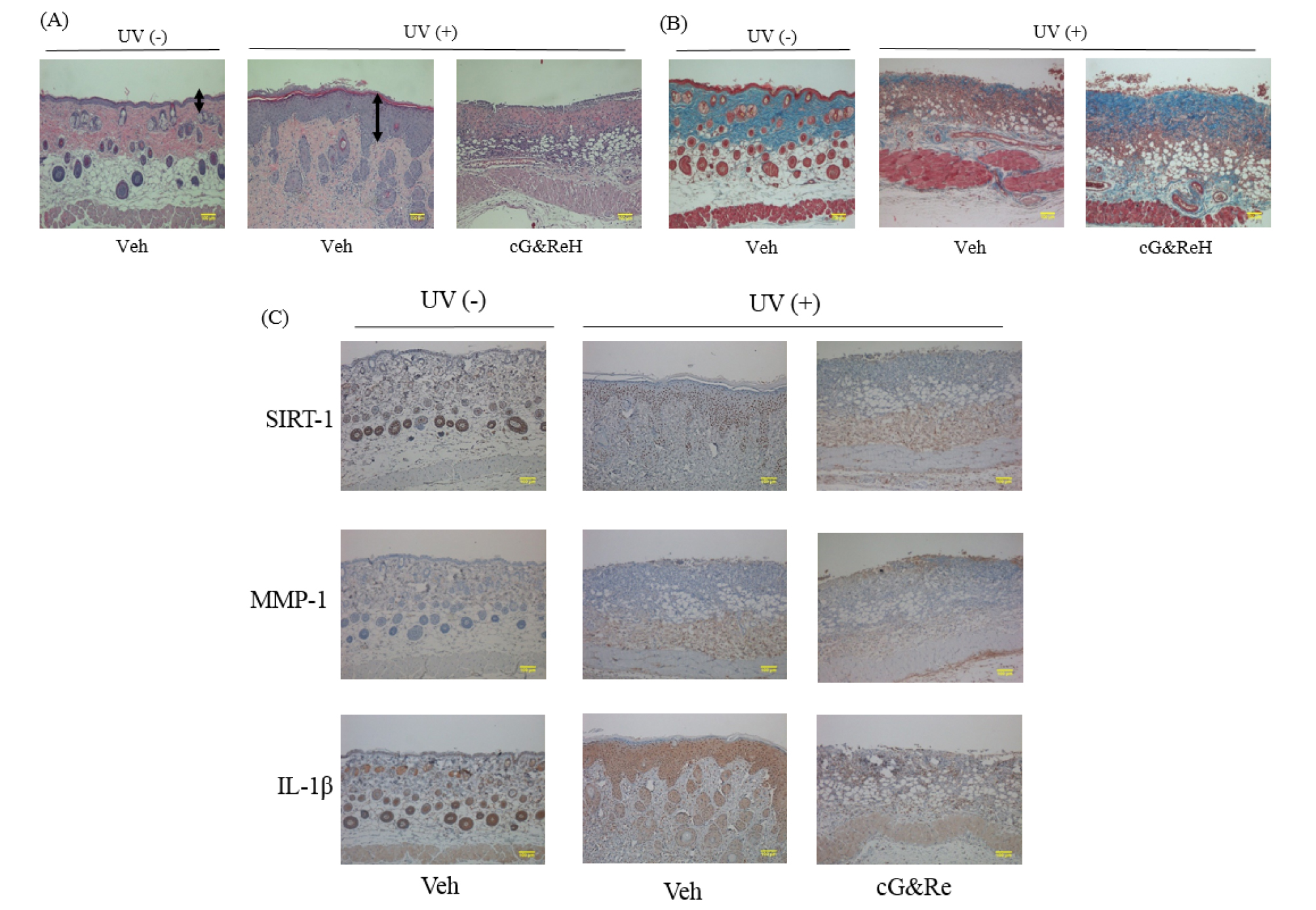
Fig. 5.
Histochemical changes and protein expression of aging-related biomarkers induced by oral administration of cG&Re. (A~B), Following oral administration of 250 ㎎/mL of cG&Re, mice dorsal skin tissues were sectioned to assess the histochemical changes related to skin thickness (A) and collagen content (B) by hematoxylin & eosin (H&E) staining and Masson’s trichrome staining, respectively. Arrows indicate the thickness of inflammatory epidermis. An asterisk depicts protected dermis. A box depicts morphology of degraded collagen. (C), SIRT1, MMP-1, and IL-1β were examined to assess anti-skin aging activity in the animal model using hematoxylin & eosin (H&E) staining. Veh, vehicle; UV (-), non UVB-irradiated; and UV(+), UVB-irradiated. Scale bar: 100 ㎜.
Discussion
There is an increasing demand for biomaterials derived from natural sources in the food and cosmetic industries (Khan and Abourashed., 2011). This demand originates from the need for better health and beauty care, with particular reference to balanced extrinsic and intrinsic beauty resulting in homeostasis (whitening, anti-inflammation, anti-skin ageing, etc.). As a result of this trend, the emergence of food ingredients based on various natural substances aimed at optimizing intrinsic beauty, has begun to attract the attention of both industry and customers. Currently, critical ageing- associated biomarkers capable of assessing specific skin- associated benefits of a food ingredient are unavailable. Therefore, many scientists have been seeking novel agents for the development of functional food biomaterials. Towards this end, the current study was intended to demonstrate the scope of cG&Re extract in alleviating UV-induced skin ageing, via downregulation of MMP and IL-1β and upregulation of SIRT-1, using mouse models.
The study indicated that cG&Re may inhibit elastase activity in a concentration-dependent manner (Fig. 1B). This activity was more compatible than that of EGCG. Therefore, we analyzed the active ingredients of cG&Re with HPLC. Because the extract did not display toxicity towards cells, we examined cell viability following UV exposure, which is a trigger of skin ageing. The findings revealed that cG&Re could protect against UV-induced cell damage in a dose- dependent manner, indicating that polyphenols, flavonoids and other small molecules may play a key role in maintaining cell viability, although the 2 major peaks at 35.5 min and 41.5 min of retention time, respectively, were not recognized as polyphenol compounds (Fig. 1A).
Mounting evidence suggests that chronic exposure to UVB can damage skin cells, enhancing skin aging (Bissett et al., 1990). Apparently, UVB (290-320 ㎚) is mostly absorbed by keratinocytes in the epidermis. It not only repeatedly induces melanin biosynthesis, but finally executes the ageing process when the skin is unable to recover from repeated exposure and/or stress responses. Up to 10 ㎍/mL cG&Re did not exert any toxic effects on human keratinocyte HaCaT cells, and restored cell viability which was reduced due to UVB- irradiation, suggesting that cG&Re is involved in inhibiting cell damage in UVB-irradiated skin cells. Photoaging is characterized by thickening, wrinkling, erythema like mottled pigmentation, skin dehydration and transepidermal water loss (TEWL) (D’orazio et al., 2013; Xu et al., 2009; Kang et al., 2018). Our studies of HaCaT cells indicated that cG&Re ultimately prevented wrinkle formation induced by UV exposure. We clearly demonstrated that MMP-1 and -13 expression was decreased by cG&Re treatment at both low and high concentrations (Fig. 4A). Because RT-PCR analysis did not indicate downregulation of any other MMPs, we surmised that the 2 MMPs, MMP-1 and MMP-13, played a key role in ameliorating the skin aging process by inhibiting the aging parameters previously described by other researchers (Vincenti and Brinckerhoff, 2002).
A new concept of the anti-aging strategy is to protect the skin after the damage has caused morphological changes in cells. Therefore, we attempted to evaluate the anti-skin aging activity of cG&Re in mouse UVB-induced models with 2 types of treatment; 1) topical application, and 2) oral administration. Topical application of cG&Re alleviated symptoms of photoaging which was confirmed numerically (Fig. 2). Our histochemical analysis demonstrated that topical application of cG&Re resulted in moderate collagen accumulation and suitable organization as loose and wavy fibers, more comparable to normal skin tissues (Fig. 3A). UV-induced damage which drives the degradation of ROS with the inflammatory response. In this study, we evaluated the role of cG&Re extracts in inflammatory responses in mice skin. Pro- inflammatory cytokine IL-1β was reported to be released and responsible for initiation of a cascade of pro-inflammatory responses following UV irradiation in HaCaT cells (Wang and Bi, 2006). IL-1β may also induce other pro-inflammatory cytokines, such as IL-6 and IL-8 (Orjalo et al., 2009). These inflammatory cytokines secreted from epidermal keratinocytes stimulate dermal fibroblasts and keratinocytes, and then upregulate mRNA and protein expressions, and enzymatic activity levels of MMPs. MMPs consequently act on collagen and elastic fibers, leading to the formation of wrinkles (Fisher et al., 2000). The mice skin tissues, stimulated with UV, showed a significant expression of low density of collagen contents in comparison with control. However, cG&Re extract exhibited a broad spectrum of inhibitory effects on the expression of IL-1β by immunohistological analysis. Therefore, in an in vivo experiment, markedly reduced IL-1β deposition were observed in cG&Re -treated UVB-induced mice, suggesting potential anti-inflammatory effect s of cG&Re. Similarly, oral administration of cG&Re also showed a strong potential for ameliorating skin aging in UVB-irradiated mouse tissues (Fig. 5A~C).
MMPs, known as zinc-containing proteinases, are involved in the degradation of ECM components, such as collagen, elastin and fibrillin-1 (Quan et al., 2009). Reportedly, inhibition of MMP production is an effective strategy for the prevention and treatment of photoaging-associated epidermal thickening and wrinkle formation induced by UV (Park et al., 2014). UVB-induced expression of MMP-1 and MMP-13 was significantly suppressed by topical application of cG& Re (Fig. 4A). MMP-1 is considered to be a major collagenase causing collagen breakdown in photoaged skin (Fisher et al., 2009). In the present study, we confirmed that cG&Re effectively inhibited not only protein expression of MMP-1/13 but also expression of MMP-1/13 mRNA, implying that MMP- 13 may be a potential indicator of the anti-skin ageing process. Human sirtuins (SIRT1-7) are known as a family of anti-skin ageing proteins, associated with cancer, Alzheimer’s disease and diabetes (Cao et al., 2009). Similar to SIRT6 and SIRT7, SIRT1 is basically localized to the nucleus, and is involved in mediating extensive activities including DNA apoptosis, mitochondrial biogenesis, hypoxia, cellular stress and inflammation, as well as homeostasis of glucogenesis and lipogenesis (Haigis and Sinclair, 2011). Absence of SIRT1 expression in cells may trigger aging-related machinery to initiate the skin aging process. Sinclare et al. reported that resveratrol in grapes overexpressed SIRT1 proteins in a rodent model, although this finding is subject to some controversy (Hubbard and Sinclair, 2014). However, there was no difference in mRNA and protein expression of SIRT- 1 between cG&Re treatment groups (Fig. 4B). In order to further investigate the molecular signaling pathway using mice tissue, we performed western blot analysis for MAPK signaling biomarkers (Fig. 4C). As predicted, phosphorylation of MAPK was dramatically reduced by the cG&Re treatment indicating that MAPKinase was associated with skin aging. Collectively, these results suggested that cG&Re triggered inhibition of MAPK signaling by inhibiting MMP-1/13 and IL-1β and activating SIRT-1.
In order to develop a functional food ingredient for anti-skin aging purposes, it is necessary to test various administration methods using animal models prior to initiating a clinical trial. This is due to the availability of many application techniques for assessing the functionality of food ingredients. Overall, this study indicated that both topical and oral application of cG&Re, protected the skin from UV-induced photo-aging in mouse models. The current study was initiated due to the need to develop functional foods from natural and nontoxic food ingredients. In addition, the findings suggest that a combination of active ingredients leading to a synergistic effect may yield better skin care while enhancing inner beauty care.




