Introduction
Materials and Methods
Preparation of plant material
Cryopreservation using droplet vitrification
Recovery assessment and plant regeneration
Flow cytometry analysis
Statistical analysis
Results and Discussion
Effect of preculture treatment on recovery rate (%) of cryopreserved shoot tips of strawberry accessions
Effect of preculture treatment on ploidy stability of cryopreserved shoot tips of strawberry accessions
Introduction
The cultivated strawberry Fragaria × ananassa DUCH is the most important berry fruit worldwide (Pinker et al., 2009). There are more than 200 accessions of strawberry cultivars in Korea (Lim et al., 2016; Rousseau-Gueutin et al., 2009). For preservation of strawberry germplasm, there always exists a possibility of contamination by runners of disease-infected plants under natural conditions. Therefore, previously, Reed and Hummer (1995) developed an alternative method termed as in vitro preservation of strawberry cultures at low temperature for medium term storage.
Maintenance of plant genetic resources in situ and ex situ has two main objectives, the conservation of genetic diversity at large and of selected varieties with agronomic and economic value (Benelli et al., 2013). Therefore, conservation plays a fundamental role in order to prevent the loss of plant species with economic and ecological importance. It is not always possible to preserve the crop under natural conditions due to possible risks of losses caused by biological and natural hazards. Cryopreservation techniques are now advanced to a stage where they can be implemented for practical storage of germplasm (Reed, 2001).
Droplet vitrification has demonstrated high recovery and established as an efficient method among the vitrification-based cryopreservation techniques, for many plant species using various explants, including shoot tips (Benelli et al., 2013; Sakai and Engelmann, 2007). Although there exist a few reports on strawberry cryopreservation (Hirai et al., 1998; Reed and Hummer, 1995; Wu et al., 1997), there are no reports on application on a large-scale involving germplasm lines.
The recently developed droplet vitrification method has been reported to be successful in many cultures e. g. Chrysanthemum, roses, potato, garlic and banana (Halmagyi and Pinker, 2006; Halmagyi et al., 2005; Kim et al., 2012; Panis et al., 2005; Yi et al., 2018). Thus, the droplet-vitrification method could be a suitable method for large scale cryopreservation of strawberries in genebanks require simple and reliable protocol. Preculture in osmotic active solutions like sucrose, mannitol or glycerol is a necessary step to prepare the plant tissue for treatment with the vitrification solution. In roses without sucrose preculture the PVS2 solution found to be immediately toxic (Halmagyi and Pinker, 2006) and that sucrose was more effective than glucose or mannitol (Halmagyi et al., 2005). This study is focused on the effect of sucrose concentration used in preculture of strawberry shoot tips regarding their recovery after freezing, including the recovery and the morphological stability of the plantlets in vitro grown.
The main objective of the present study was to develop an efficient and stable method of cryopreservation via droplet vitrification for shoot tips of the two cultivars of strawberry ‘Massey’ and ‘MDUS3816’ and to achieve high recovery of regrowth rate from cryopreserved explants.
Materials and Methods
Preparation of plant material
In vitro grown strawberry (Fragaria × ananassa Duch.) accessions, ‘Massey’ (released variety) and ‘MDUS3816’ (wild relative) were used in this study after multiplication from runner tips. In vitro stock shoots were maintained in shoot multiplication medium composed of Murashige and Skoog (1962) medium supplemented with 2.0 ㎎/L 6-benzylaminopurine (BA) + 0.1 ㎎/L 1-naphthaleneacetic acid (NAA) and 30 g/L sucrose + 2.6 g/L phytagel (Duchefa Biochemie B.V., The Netherlands). The pH of the medium was adjusted to 5.8 before autoclaving at 121℃ for 15 min. The stock cultures were maintained at 24 ± 1℃ under a 16 h photoperiod with a light intensity of 50 µmol s-1m-2 provided by cool-white fluorescent tubes. Subculturing was done at every 4 weeks intervals. Explants used for experiments were excised from 6weeksold plants as previous study (Lee et al., 2019).
Cryopreservation using droplet vitrification
The protocol of cryopreservation and pre/post treatment was followed previous experiments (Lee et al., 2019) as showed in Fig. 1. Shoot tips (~ 1-2 ㎜ in length) were excised from 8weeks old in vitro grown plantlets using sterile forceps and scalpel under a stereo microscope under aseptic conditions. The cultures were cold-hardened in dark at 4℃ for various time periods ranging from 0 to 6 weeks and precultured in MS medium containing 0.3 ~ 0.7 M sucrose. The cultures were incubated for different time durations at 25℃. The various preculture treatments are as follows: 0.3 M sucrose for 30 h, followed by 0.5 M sucrose for 16 h, and 0.3 M sucrose for 30 h followed by 0.7 M sucrose for 16 h. Precultured shoot tips were osmoprotected with a loading solution (LS, C4) for 40 min. Loading solution (LS, C4) (Kim et al., 2009b) comprised MS medium containing 35% of PVS3 (17.5% of glycerol + 17.5% of sucrose) without DMSO. Apparently, the loaded shoot tips were exposed to a dehydration solution (B1) (Kim et al., 2009a) containing 100% of PVS3 (50% of glycerol + 50% of sucrose) for 60 min at 25℃. The shoot tips were transferred onto droplets containing 2.5 µL PVS3, which were placed on a sterilized aluminum foil strip (4.0 ㎝ × 0.5 ㎝, Fig. 1e), followed by direct immersion in LN. The shoot tips were removed from the LN after 30 min and transferred into 2 mL cryotubes filled with LN for cryostorage for 1 h. Aluminum foils with shoot tips were removed from LN and immediately transferred into an unloading solution containing liquid MS with 0.8 M sucrose at room temperature for 40 min.
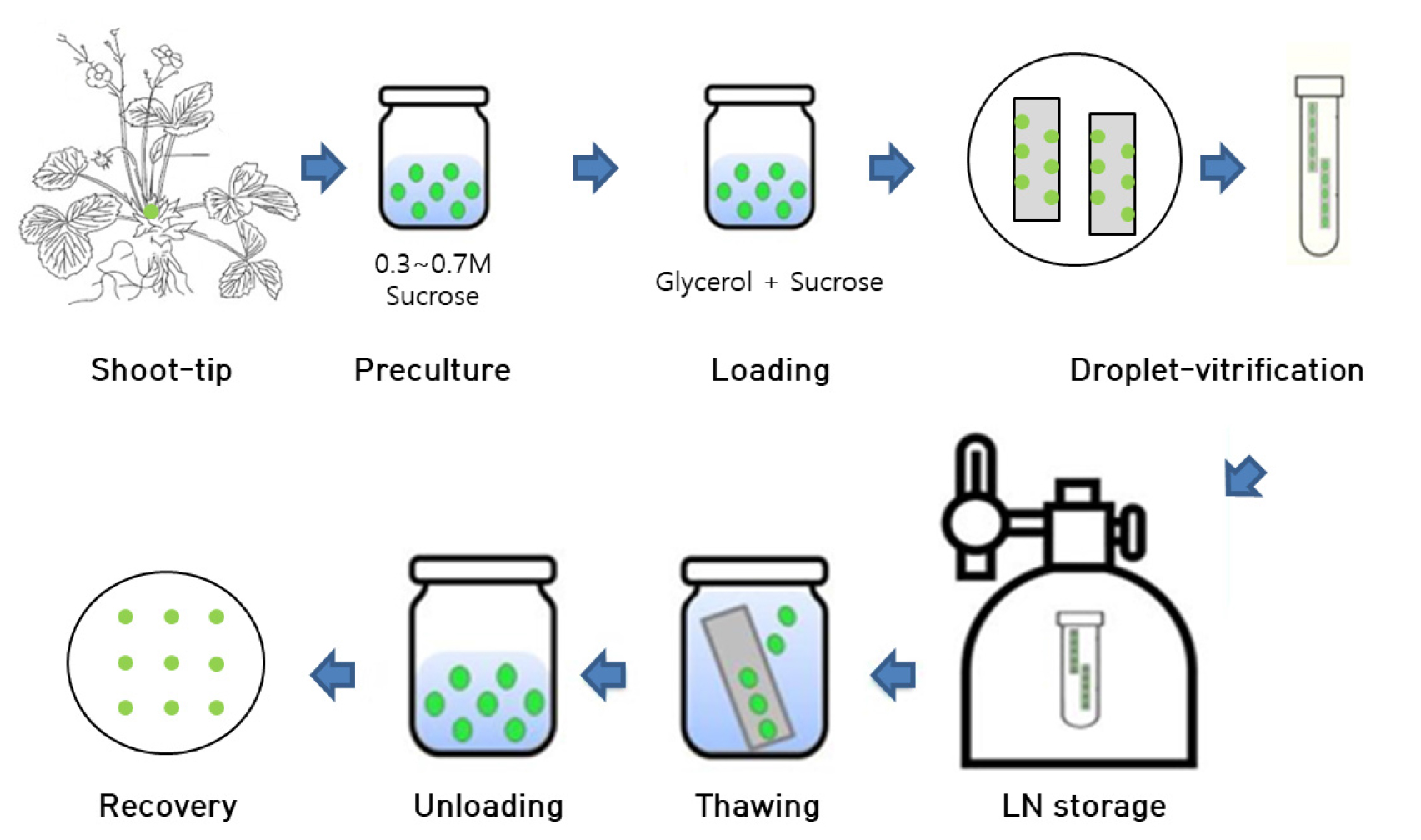
Fig. 1
Cryopreservation procedure on shoot tips of Strawberry (Fragaria × ananassa) by droplet-vitrification. Source of in vitro grown strawberry plantlets used for shoot tips collections. Position of the shoot tips on the plantlets (marked with green color) from which shoot tips are excised. The excised shoot tips used for cryopreservation experiment. Shoot tips are treated with loading solution (LS, C4) for 40 min at 25℃. Dehydrated with PVS3 (B1) for 60 min. Dehydration of shoot tips in 2.5µL PVS3 droplets carried on sterilized aluminum foil strips. Immersion of aluminum foil along with shoot tips into the liquid nitrogen (LN) for 1 h. Cryopreserved shoot tips were unloaded in MS+0.8 M sucrose for 40 min at 35℃ for thawing. Inoculating the cryopreserved shoot tips on post-thaw culture medium containing NH4NO3-free MS medium with GA3 1.0 + BA 0.5 for 5 weeks and later cultured on MS medium with BA 0.5 for 9 weeks.
Recovery assessment and plant regeneration
The cryopreserved, unloaded shoot tips were post-thaw cultured in Petri dishes (90 ㎜ in diameter) for shoot recovery in the presence of different post-cultured media under standard conditions, as described by Lee et al. (2011) and Wang et al. (2014). Rewarmed shoot tips were post-cultured in NH4NO3-free MS medium containing 3% sucrose + 1.0 g/L casein + 1.0 ㎎/L GA3 + 0.5 ㎎/L BA for shoot recovery until 5 weeks followed by transferring to normal MS medium containing 3% sucrose + 1.0 g/L casein + 0.5 ㎎/L GA3 until 9 weeks. Subsequently, the plantlets were transferred to hormone free-MS medium. After attaining the height of 8-10 ㎝, the plantlets were transferred to greenhouse for hardening. Filter-sterilized GA3 was added to the medium after autoclaving, while BA was directly added to the medium before autoclaving. The cultures were placed at 24 ± 1℃ in the dark for 1 day and then transferred to light conditions for recovery. All the cultures were grown in petri dishes for 5 weeks, and later cultured in bottles. Shoot regrowth was defined as percentage of shoot tips regenerating into shoots with the development of leaves after 4-6 weeks of post-thaw culture. Development of shoots along with roots was observed after 8 weeks in hormone-free MS medium. Consequently, fully in vitro grown plantlets were transferred to soil under greenhouse conditions for acclimatization.
Flow cytometry analysis
For ploidy analysis, flow cytometry method was applied using the Cyflowploidy analyzer (Sysmex corporation, Germany). Nuclei suspension of each accession was made by placing leaf discs of 0.5㎠ from young plant after each treatments in a Petri plate and 500 μL extraction buffer (Cystain UV Precise P Extraction Buffer, 2.5 μM polyvinylpyrrolidone (PVP), 10.12 mM dl-Dithiothreitol). Tissue disruption was made by manually chopping the samples using a sharp razor blade. An additional 500 μL of extraction buffer was poured to the chopped tissue and the suspension was passed through a 50 μm Cell Trics nylon mesh filter (Sysmex, Billerica, MA). The nuclei suspension was stained by adding 2 mL of staining buffer (Cystain UV Precise P Staining Buffer), and by incubating the samples at room temperature for 2-5 minutes. The samples were analyzed by flow cytometry as above.
Statistical analysis
In cryopreservation experiments, shoot tips receiving all treatments but without freezing in LN served as the treated control (-LN), while cryopreserved (+LN) shoot tips served as test material. At least 10-12 shoot tips (replicates) were used for each treatment. All the experiments were conducted twice. Results are presented as means with their standard error (SE). The data were analyzed (mean ± SE) from two experiments, and least significant differences (LSD) were calculated at P < 0.05.
Results and Discussion
Effect of preculture treatment on recovery rate (%) of cryopreserved shoot tips of strawberry accessions
The sucrose concentration and time duration of preculture treatments had no significant effect on the recovery rate (%) of treated control (-LN) of the shoot tips in both the accessions (‘Massey’ and ‘MDUS3816’); however, significant effect was observed on the recovery rate of cryopreserved (+LN) shoot tips of the two accessions of strawberry (Table 1). The recovery rate (%) was higher from the shoot tips of the treated control (-LN) than from the cryopreserved (+LN) shoot tips in both the accessions. For cryopreserved (+LN) shoot tips, the highest regeneration rate (%) in both cultivars was obtained when the shoot tips were exposed to the preculture treatment (0.3M sucrose for 30 h + 0.5 M sucrose for 16 hat 25℃). On the other hand, upon exposure of the shoot tips to increased concentration of sucrose (0.7 M), a decrease in the recovery rate (%) was observed, thus demonstrating that standardization of sucrose concentration plays an important role during preculture treatment of the explants for cryopreservation protocol. The highest recovery rates (%) were 65.5% and 50.0 % for cryopreserved shoot tips of ‘Massey’ and ‘MDUS3816’, respectively. The recovery rate (%) was lower for both treated (-LN, +LN) shoot tips of accession ‘MDUS3816 (wild relative)’ compared with the recovery rate of accession ‘Massey (released variety)’, which can be explained as sensitivity of each accession to osmotic or low temperature stress. For accession ‘MDUS3816’, recovery rates (%) of shoot tips cryopreserved (+LN) were stable in every 2 week-investigation while the recovery rates (%) of treated control (-LN) were increased with the time course of post culture (Fig. 2), which can be assumed that tissue of wild relative accession ‘MDUS3816’ is osmotically tolerant comparative as sensitive to low temperature.
Table 1.
Effect of preculture treatment on recovery rates of strawberry shoot tips before (-LN) and after cryopreservation (+LN)
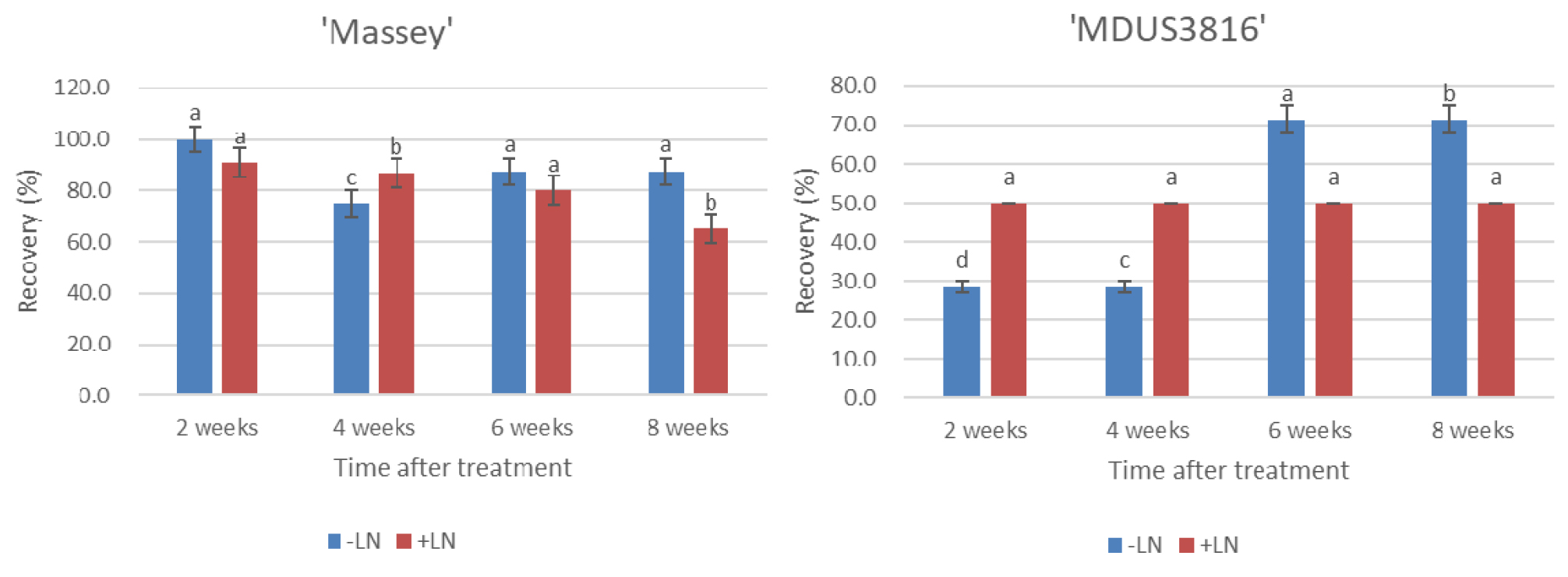
Fig. 2
Effects of preculture condition of 0.3 M and 0.5 M sucrose combination on recovery rate (%) of the treated control (-LN) and cryopreserved (+LN) shoot tips of the two accessions of strawberry (‘Massey’ and ‘MDUS3816’) by droplet vitrification. Shoot tips excised from 8-week-old in vitro grown cultures were precultured with MS+0.3 M and 0.5 M sucrose for 30 h and 16 h respectively. Precultured and loaded shoot tips were exposed to loading solution (LS, C4) for 40 min and dehydration solution (B1) for 60 min prior to direct immersion in LN. Cryopreserved shoot tips were unloaded in MS+0.8 M sucrose for 40 min at 35℃ for thawing. Subsequently, the plantlets were transferred to hormone free-MS medium. The plantlets after attaining 8-10 cm height were transferred to greenhouse conditions for acclimatization. The regrowth rate (%) of the cryopreserved shoot tips was recorded every 2 weeks after incubation at 25℃. Each treatment contained 15 replicates. The results are presented as means ± SE. Bars with the same letters are not statistically different from each other according to the least significant difference (LSD) (P = 0.05).
Exposure of in vitro grown explants to preculture treatment in the presence of various sucrose concentrations is known to enhance the survival rate (%) from cryopreserved shoot tips. Previous reports by various researchers have displayed the effect of various sucrose concentrations on recovery rate (%) during preculture which acts as supporting evidence to our research. Yamamoto et al. (2012) employed 0.8 M sucrose in preculture treatment for 48 h for strawberry shoot tips, and Niino et al. (2003) observed difference in the shoot regrowth during the preculture of strawberry shoot tips at various concentration of sucrose in the preculture medium. Similarly, Wang et al. (2014) reported that in Chrysanthemum morifolium, the highest shoot regrowth rate (75%) was obtained when shoot tips were precultured with 0.5 M sucrose for 24 h. Both lower (0.25 M) and higher (0.75 M) sucrose concentration resulted in marked reduction in shoot regrowth rate. On the other hand, our results were in agreement with the results reported by Pinker et al. (2009) in their study on strawberry using droplet vitrification method, which displayed that the highest recovery rate (60%) was achieved after preculture with 0.25 M sucrose for 24 h. These reports showed that sucrose concentration and incubation plays an important role in recovery of shoots. The employed sucrose concentrations and incubation periods in this study were based on our recent studies with different cultivars (‘Gurumi40’, ‘Wonkyo3114’) of Fragaria × ananassa Duch. (Lee et al., 2019) and C. morifolium (Yi et al., 2018).
Effect of preculture treatment on ploidy stability of cryopreserved shoot tips of strawberry accessions
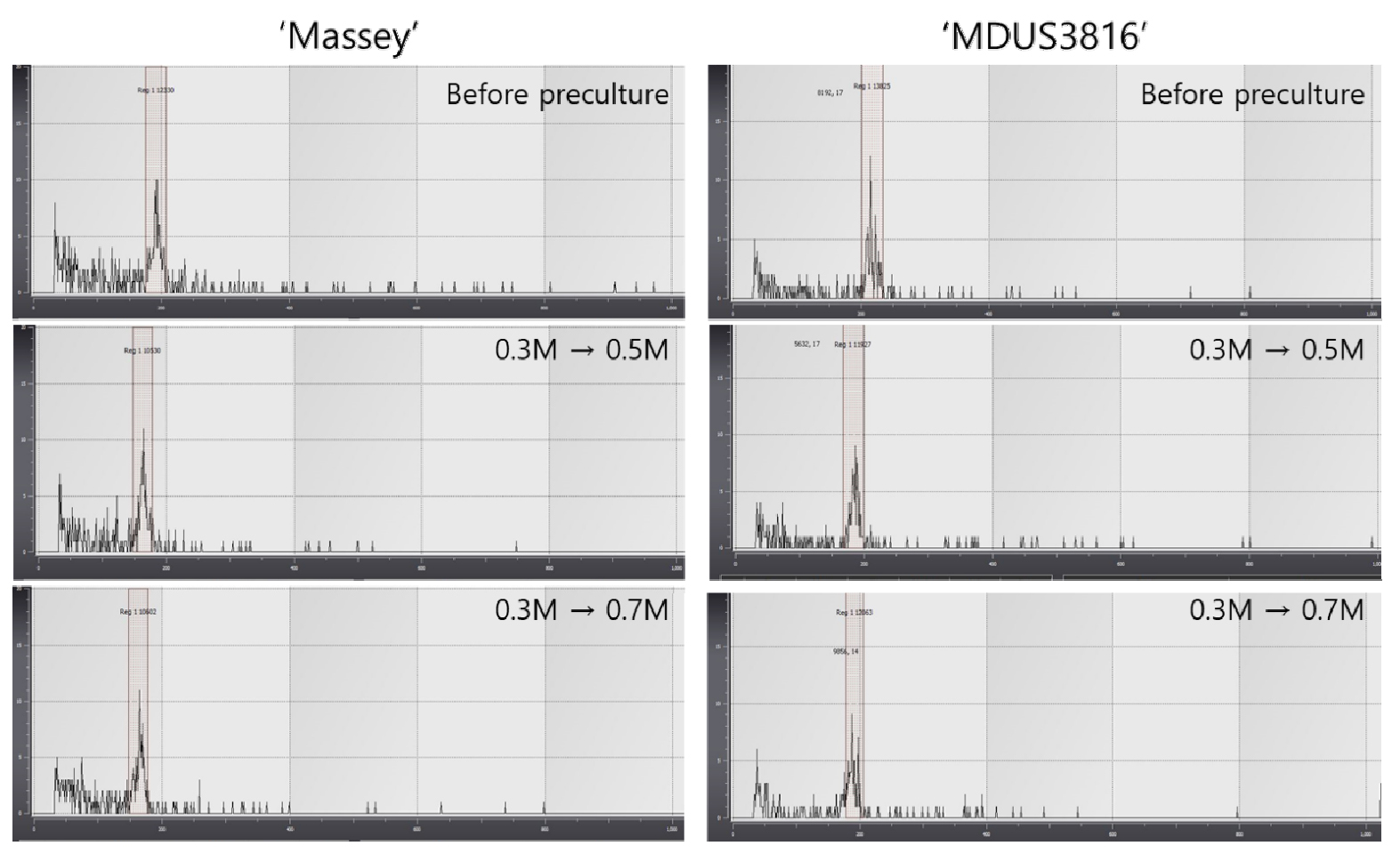
In several researches, it was reported that repeated micro propagation and preculture with high concentration of sucrose solution could induce off-type of ploidy and phenotype, respectively in strawberry germplasm (Pinker et al., 2009; Sansavini et al., 1990). In this study, 0.3-0.7 M of sucrose solution were applied to preculture for cryopreservation of shoot tips on in vitro grown strawberry which were investigated for ploidy analysis through flow cytometry analyzer. Pinker et al. (2009) observed that the number of off-types in the field was affected by the sucrose concentration. After preculture in 0.1 M sucrose no off-types were observed, while after preculture in high sucrose concentrations the number of off-types was much higher reaching 20% at the highest sucrose concentration.
The results of our study showed each peak at the constant range which means no variation in ploidy level by preculture and cryopreservation treatments (Fig. 3). As Fig. 4 indicated, morphologically there was no significant variation among plantlets cryopreserved with preculture treatment (0.3 M sucrose for 30 h + 0.5 M sucrose for 16 h at 25℃).

Fig. 4
Recovered plantlets from strawberry (accession of ‘MDUS3816’) shoot tips for each treatments of cryopreservation with preculture condition of 0.3 ~ 0.7 M sucrose. Inoculating the cryopreserved shoot tips on post-thaw culture medium containing NH4 NO3-free MS medium with GA3 1.0 + BA 0.5 for 5 weeks and later cultured on MS medium with BA 0.5 for 9 weeks.
Thus, the droplet vitrification protocol is a practical and stable technique for cryopreservation of strawberry germplasm and represents a promising method for the cryopreservation of other plants with slight modifications. Further studies are necessary to confirm the genetic stability of mature plants regenerated by biochemical and molecular analyses constantly.