Introduction
Materials and Methods
Reagents and standards
Plant materials
Extraction, separation, and analysis of sesquiterpene lactones (SLs)
Statistical analyses
Results and Discussions
Introduction
Lettuce is the highest ranked vegetable in production and economic value, and only second to potato in per capita consumption worldwide (Kim et al., 2018). Lettuce grows annually in backyards, containers, shade net, greenhouse, and also using hydroponics. Lettuce is grown as leaf vegetable as well as for its stem and seeds. It is one of the most important ready-to-use products and has been principally consumed as salad, a “wrap-up vegetable”, as cooked vegetable, and in sandwiches (Martínez-Sánchez C 2012; Seo et al., 2009; Cho et al., 2016). Lettuce varieties differ in: head shape (crisphead, butter head, cos (romaine), and leafy); leaf color (green, red/green, red, purple, and green/purple); and leaf shape (could be circular, broad, elliptic, and transverse). Among the various pharmacologically significant phytoconstituents of lettuce, sesquiterpene lactones (SLs) are one of the important secondary metabolites significant to plants and human (Rees & Harborne, 1985; Chadwick et al., 2013; Tamaki et al., 1995). Other sources of SLs include, beverages such as chicory root tea, spices such as star anise, and herbs (Chadwick et al., 2013). SLs are C-15 terpenoids that naturally occur in the form of hydrocarbons, alcohols, ketones, aldehydes, acids or lactones (Graziani et al., 2015). Lactucin and lactucopicrin, the major SLs reported in lettuce, could contribute significantly to the bitterness nature of lettuce cultivars (Price et al., 1990; Seo et al., 2009). The content of SLs was found to exhibit a significant variability based on the variety in chicory and endive (Ferioli et al., 2015). Growing seasons have been reported to influence the concentration of SLs in forage chicory cultivars, keeping in mind the specific response depended on the type of cultivar (Foster et al., 2011). Sensory properties including, bitterness, appearance, flavor, texture, and overall acceptability and total phenolic properties of lettuce are also dependent on the growing season (Bunning et al., 2010). Genetic improvement of crops partly depends on the existence of variation in useful phytochemical traits within germplasm and SLs are one of the important traits for developing a new variety of lettuce (Sung et al., 2016, Jang et al., 2013). Sweeter taste and more crispy texture of lettuce are the favorable sensory attributes for consumers (Pollard et al., 2002). The SLs found in leaves and flowering heads of plants are sometimes available in a range of cell types, produced in high levels constantly, or in some species they may be held in storage organs (Chadwick et al., 2013). Knowledge of the profiles of sesquiterpene lactones in different growing seasons and in individual organs of the plant is important. Seo et al. (2009) had reported the content of SLs in basal leaves, mid-stalk leaves, and flower stalk leaves, from a single cultivar. Yet, a comprehensive understanding of variation in the content of SLs between different tissues of lettuce and growing seasons is still lacking.
This study was aimed to investigate the variation of two major bitter SLs (lactucin and lactucopicrin) content in different tissues (inflorescences, upper leaf, middle leaf, lower leaf, upper stem, middle stem, and lower stem) of the reproductive stage of lettuce harvested in spring and autumn seasons. As SLs accumulation partly represents the net effect of sensory properties of lettuce, accurate knowledge of the accumulation profile in different organs and seasons provides a baseline data for breeding and also helps in explaining the role of these compounds in interactions with herbivores and pathogens.
Materials and Methods
Reagents and standards
All chemicals and solvents used in extraction and analysis were of analytical grade and purchased from Fisher Scientific Korea Ltd. (Seoul, South Korea) and Sigma-Aldrich (St. Louis, MO, USA). Standards (lactucin and lactucopicrin) were HPLC grade (> 95% purity) and purchased from Extrasynthese (Lyon, France).
Plant materials
Lettuce was grown at the research farm of the National Agrobiodiversity Center (NAC), Jeonju (35°49′18″ N 127°08′56″ E), Republic of Korea. Seeds of nine lettuce accessions were sown in plug trays during the autumn (2016) and spring (2017) seasons, and seedlings were grown in a greenhouse. Four-week-old seedlings were transplanted to the field using hydroponics in a plastic house on 20th of September (2016) and 19th of March (2017). Planting density was 20 x 20 ㎝. Rural Development Administration (RDA)’s recommendation for the cultural management practices of lettuce were followed in the field. Each accession was consisted of 24 plants. Plant growth was maintained using nutrient solution throughout the growing season. Qualitative morphological characteristics were recorded on the basis of the standard lettuce descriptors for Protection of New Varieties of Plants (UPOV, 1981). The description of some selected qualitative characters of lettuce is given in Table 1. The qualitative characters were recorded based on plant observation on field when plants of each variety completed flowering.
Table 1. Morphological characteristics of lettuce varieties
Extraction, separation, and analysis of sesquiterpene lactones (SLs)
Each of the nine lettuce varieties consisting of 24 individuals were harvested and dissected into pedicel, upper leaf, middle leaf, lower leaf, upper stem, middle stem, and lower stem, placed in vinyl freezer bags and held at -80°C. The frozen samples were subsequently lyophilized for 48 hr using LP100 vacuum freeze-drier (Ilshibiobase, Rijssen, Netherlands). The freeze-dried samples were then ground to a fine powder using a mortar and pestle, and held at -80°C until analysis. Samples were extracted based on the method described by Price et al. (1990) with some modification. Briefly, powdered lyophilized lettuce (0.25 g) was extracted with 100 ㎖ of methanol by boiling under reflux at 65°C for 1 hr and 20 min and filtered through Whatman #2 filter paper. The methanol was evaporated under reduced pressure using High Capacity Centrifugal Evaporator (Genevac, HT-4X, 5 mm Hg, 30 -35°C). The crude extract was then partitioned two times between water/chloroform (20 ㎖; 1:1 mixture by volume) with the chloroform separated, dried over anhydrous magnesium sulfate, and evaporated using a High Capacity Centrifugal Evaporator (Genevac, HT-4X, 5 ㎜ Hg, 30~35°C). The residue was dissolved in 0.4 ㎖ methanol/chloroform (1:2, v/v) and the SLs separated using high-performance liquid chromatography (HPLC).
The sesquiterpene lactones were analysed using Agilent 1260 infinity high-performance liquid chromatography (HPLC) system equipped with UV visible Diode Array Detector (SPD-M10A). A Phenomenex Luna C18 (250 x 4.6 mm, 5 ㎛) column connected to a Phenomenex Security Guard (ODS Octadecyl, 4 x 3.0 ㎜) was used for separation of SLs. The column thermostat was maintained at 30°C. The solvent system was comprised of water (mobile phase A) and acetonitrile (mobile phase B). The elution program was as follows: 0-3 mins, 10% B; 5-15 mins, 35% B; 15-25 mins, 35-100% B, and 25-30 mins, 100% B. The SLs were monitored at a detection wavelength of 256 ㎚. The flow rate and injection volume were kept at 0.8 ㎖/min and 20 ㎕, respectively. Quantification was done using calibration equations (lactucin, Y= 3938.6 X - 4.7184 R2= 1; lactucopicrin, Y= 2606.8X + 1.2828, R2= 1) derived from the calibration curves of the corresponding standards.
Statistical analyses
Results were expressed as means ± standard deviation (SD) of nine measurements three from biological replicates and three tests, on a dry weight basis. Data were analyzed using one-way analysis of variance (ANOVA) followed by Duncan’s multiple range test using SPSS V.17.0 statistical program (SPSS Inc., Chicago, USA). Significant level was set at p < 0.05.
Results and Discussions
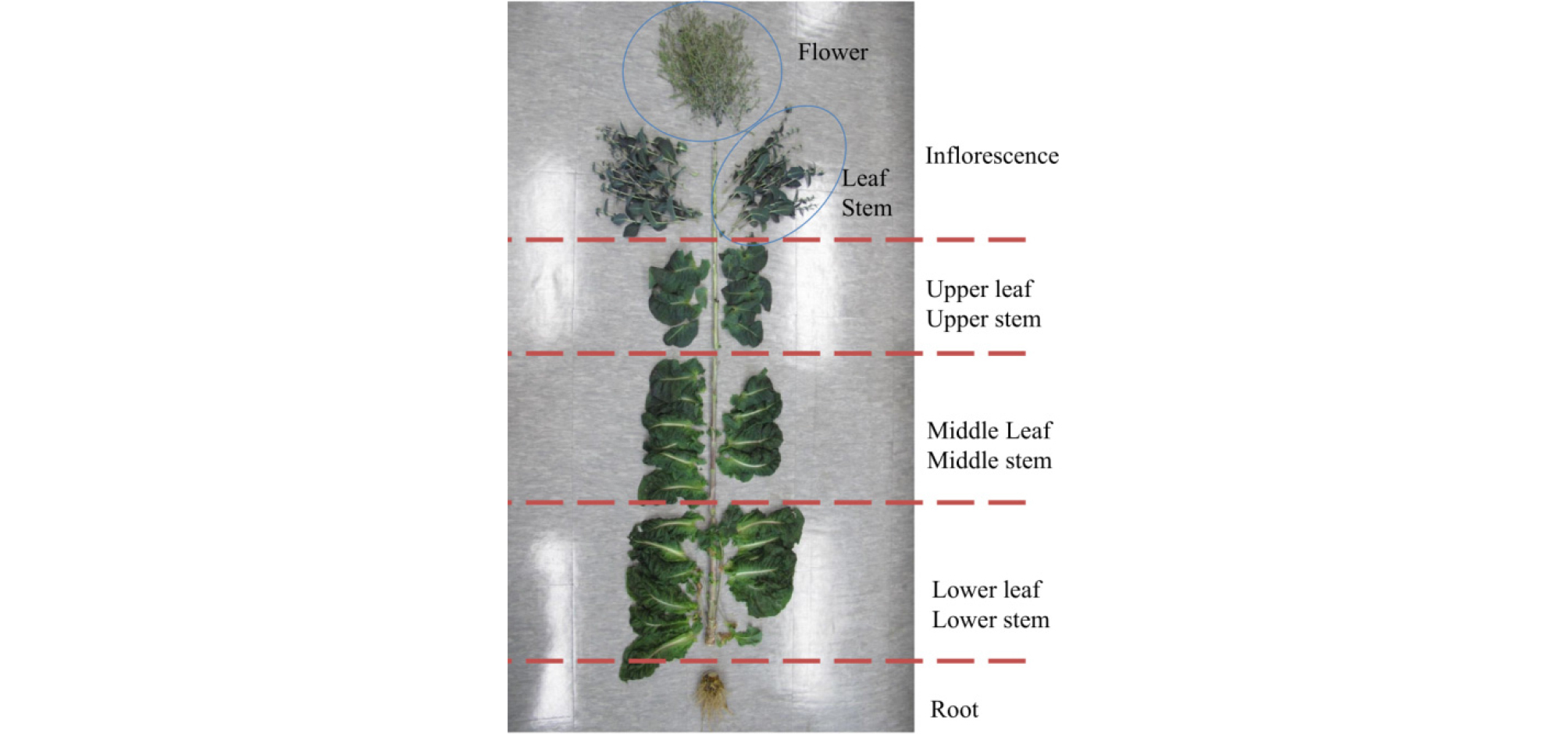
This study was designed to investigate the patterns of variation in SLs concentration in nine lettuce cultivars with respect to plant part and cultivation season. A representative sample was prepared from each cultivar which had 24 individual lettuce plants. Samples were taken from the major organs at the reproductive stage, when the elongated stem has produced inflorescences and flowers are present. Each plant was segregated into inflorescence, upper leaf, middle leaf, lower leaf, upper stem, middle stem, and lower stem as shown in Fig. 1.
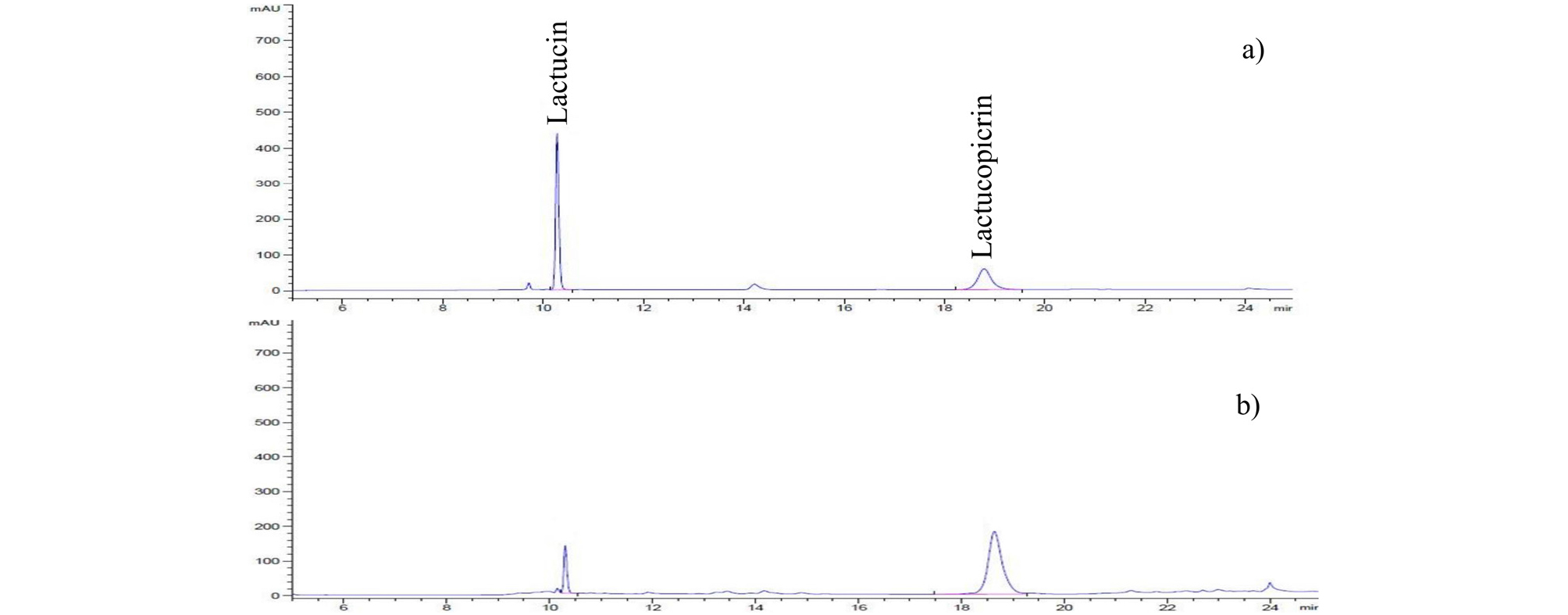
The mean SLs concentrations of different organs of lettuce plant cultivated in spring and autumn seasons, expressed in microgram per gram of dry weight (㎍/g DW), are given in Table 2. A representative HPLC chromatogram showing authentic standards (lactucin and lactucopicrin) and a randomly selected lettuce sample is presented in Fig. 2. Analysis of variance (ANOVA) indicated that individual SLs as well as total SLs concentration showed a significant inter-cultivar and intra-plant variations. The concentration of total SLs in various tissues of lettuce cultivated in spring season varied from 5.7 to 22.5 fold ranging from as low as 27.4 ㎍/g DW in the upper stem (cultivar “PI 176588”) to as high as 2,292.0 ㎍/g DW in the inflorescence (cultivar “709849-1”). During autumn cultivation, the concentration of SLs varied from 2.0 to 14.4 fold ranging from as low as 32.4 ㎍/g DW in the lower stem (cultivar “PI176588”) to as high as 838.0 ㎍/g DW in the upper leaf (cultivar “Dambaesangchu”). In almost all the samples considered in this study, the concentration of lactucin and lactucopicrin were significantly higher in the inflorescences compared to other parts. The total SLs in in the inflorescences ranged from 457.7 to 2,292.0 ㎍/g DW and 141.1 to 768.6 ㎍/g DW in spring and autumn seasons, respectively. Intermediate levels were observed in upper leaves (205.8 to 1,222.8 ㎍/g DW, spring cultivation; 37.1 to 838.0 ㎍/g DW, autumn cultivation) and upper stem tissues (27.4 to 396.9 ㎍/g DW, spring cultivation; 43.5 to 546.1 ㎍/g DW, autumn cultivation), and lesser amounts in lower stem and leaves.
Table 2. The concentration of SLs in various tissues of lettuce cultivars and two cultivation seasons
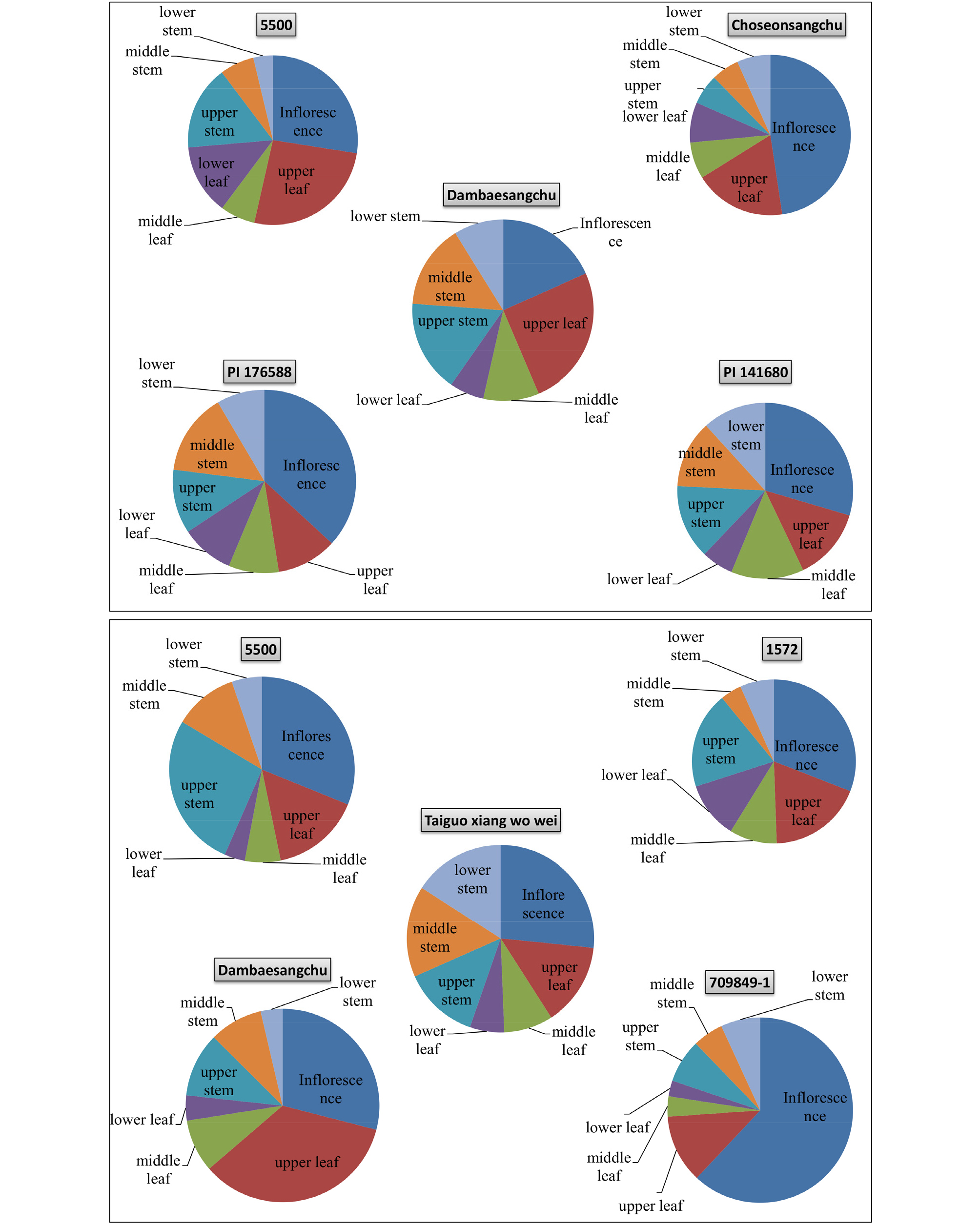
To better visualize the SLs accumulation in various organs of lettuce, two-dimensional pie charts for selected representative lettuce varieties are presented in Fig. 3, where the area of the sectors are proportional to the concentration of SLs. The concentrations of the SLs (lactucin + lactucopicrin) in roots and different parts of inflorescence (flower, leaf, and stem) of cultivar “709849-1”, which had the highest SLs in its inflorescence, were further evaluated. The results were 1416.7 ± 55.1, 533.2 ± 39.4, 342.1 ± 34.0, 441.4 ± 16.6, 280.8 ± 15.1, 130.3 ± 197.2, 101.7 ± 10.7, 254.6 ± 4.2, and 164.6 ± 5.2 ㎍/g DW in flower, leaf in inflorescence, stem in inflorescence, upper leaf, upper stem, middle leaf, middle stem, lower leaf, lower stem, and root parts, respectively.
To assess the effect of cultivar and season of cultivation on SLs concentration, a combinative consideration of results were taken into account, calculating average values of SLs in various tissue samples within each cultivar. Higher lactucin (1.2 to 5.6 fold) and lactucopicrin (1.1 to 3.9 fold) concentration was observed in spring compared to autumn cultivation in all samples except in cultivars “5500” and “709849-1”, which showed 1.9- and 1.2-fold higher concentration of total SLs, respectively. The concentrations of lactucin and lactucopicrin cultivated in spring season varied 4.6 and 3.3 fold ranging from 12.8 (cultivar “Taiguo xiang wo wei”) to 59.0 (cultivar “5500”) ㎍/g DW and 145.8 (cultivar “PI 176588”) to 482.9 (cultivar “709849-1”) ㎍/g DW, respectively. The concentration of lactucin and lactucopicrin in autumn season cultivation ranged from 6.0 (cultivar “PI 176588”) to 61.7 (cultivar “5500”) ㎍/g DW and 44.1 (cultivar “1572”) to 454.5 ㎍/g DW (cultivar “Dambaesangchu”), respectively.
Plants accumulate phytochemicals, including SLs, in all their vegetative and reproductive parts. However, the content of SLs in individual lettuce organs varies significantly. Generally, the concentration of SLs increases from lower part to the upper of the plant. These patterns are in accordance with a previous report (Seo et al., 2009). SLs could function in defense against herbivores and pathogens because of the bitter taste repelling chewing insects and birds (Chadwick et al., 2013; Mithöfer & Boland, 2012). The pattern of differences of SLs among organs in lettuce is consistent with predictions of optimal defense theory which states that defense should be preferentially allocated to more valuable parts with high probability of attack (Zangerl & Bazzaz, 1993). Inflorescences which contain the reproductive organ, such as flowers, contribute most to plant fitness and hence expected to contain the highest content of defense compounds. The concentration of SLs in lettuce tissues also depends on stages of development, where younger plant organs (small leaves & stems in inflorescences and upper leaves & stems) relatively have higher concentration of SLs compared to older plant organs (middle/lower leaves and stems).
Like the position of the various organs of lettuce, cultivar and harvesting date influenced the concentration of individual as well as total SLs. Our results were in accordance with the report of Foster et al. (2011), where SLs concentration significantly varied in forage chicory cultivars. These authors have also reported that concentration of SLs was lower during autumn than in late spring and early summer. Bitterness of lettuce cultivars, which has been partly linked to the presence of sesquiterpene lactones (notably lactucin and lactucopicrin) (Price et al., 1990), was found to increase with temperature (Bunning et al., 2010).
In conclusion, we have investigated the pattern of variability of SLs content in various tissues of lettuce. Generally, the concentration of SLs in various organs of lettuce increases as one goes upward from the base. A more detailed physiological study to determine the effect of synthesis, transport, and degradation of SLs to its variability in plant distribution is needed. Lettuce cultivars harvested in spring had relatively accumulated higher SLs compared to autumn harvest. Moreover, the study showed a wide variation in SLs contents among lettuce varieties. This study focused mainly on SLs quantification of nine lettuce varieties. Aside from this, molecular characterization is recommended for further understanding of their genetic variation. As lactucin and lactucopicrin are the major SLs which affects the sensory property of lettuce, their quantitative variation in the lettuce cultivars is useful for breeding new varieties with better consumer acceptance.