Introduction
Materials and Methods
Plant material
Cryopreservation procedures using droplet vitrification
Preculture
Loading
Dehydration
Unloading
Statistical Analysis
Results
Effects of preculture treatments on regrowth rates of non-cryopreserved (-LN) and cryopreserved (+LN) shoot tips from two freesia cultivars
Effects of loading and dehydration treatments on regrowth rates of non-cryopreserved (-LN) and cryopreserved (+LN) shoot tips from two freesia cultivars
Effects of unloading treatments on regrowth rates of non-cryopreserved (-LN) and cryopreserved (+LN) shoot tips from two freesia cultivars
Discussion
Introduction
Freesia (Freesia hybrida) is an ornamental cormous plant belonging to the Iridaceae family (Pourkhaloee and Khosh-Khui, 2015). It is native to South Africa but has gained popularity and cultivation worldwide due to its attractive qualities such as pleasant fragrance, long vase-life and the wide range of flower colors (Gao et al., 2010; Li et al., 2020). However, despite its economic potential, freesia plants are susceptible to various pathogens, particularly viruses, which affects the quality and yield of the plants (Choi et al., 2016). To overcome this challenge and ensure the production of healthy plants, efforts have been made to develop virus-free plants using tissue culture techniques. Tissue culture can help produce healthy plants and reduce the risk of germplasm loss. However, there have been concerns about the occurrence of deformed flowers in subsequent subcultures during tissue cultures, which can affect the overall quality of the plants (Kang et al., 2017). This issue highlights the need to establish reliable methods for long-term conservation of freesia germplasm while maintaining its integrity.
Cryopreservation has been practiced as a safe and efficient technique for the long-term preservation of various crops, especially those that are vegetatively propagated (Kulus and Zalewska, 2014; Wang et al., 2021). This method involves freezing plant tissues such as shoot tips, callus, pollen and somatic embryos in liquid nitrogen at an extremely low temperature (-196℃), allowing them to be stored without any changes for an unlimited period of time (Mubbarakh et al., 2014). There are two main types of cryoinjury that need to be avoided during cryopreservation: intra- and extracellular ice crystallization (Engelmann, 2011). Both types of ice formation can lead to a damage to cell structures and functions. To prevent ice crystal formation, cryoprotective solutions (known as cryoprotectants, CPAs) are used to protect the cells from the harmful effects of freezing, which play significant roles in successful cryopreservation. However, high concentrations of CPAs can be toxic to cells if not properly managed (Kim and Popova, 2023). Therefore, a careful optimization of cryoprotectant concentrations and exposure times is essential to achieve successful cryopreservation.
In the process of cryopreservation, various steps are followed to ensure the successful preservation of plant samples. These steps include preculture, cryoprotective treatment, storage in liquid nitrogen (LN), rewarming and recovery. Each step plays a crucial role in maintaining the viability of the samples and their ability to regrow after cryopreservation. For some plant species cryopreservation protocols have been successfully established, others may be more sensitive to the stresses involved in the process (Popova et al., 2023). During cryopreservation, plant tissues are exposed to various stresses, such as physical, mechanical, and oxidative stresses, which can affect their regenerative capability after successive steps in a cryopreservation protocol. Therefore, to improve the regenerablity of the cryopreserved materials, it is important to optimize the conditions of the different steps in the cryopreservation protocol (Kaczmarczyk et al., 2011).
To the best of our knowledge, no reports have been published on the cryopreservation of Freesia hybrida in the past two decades. Merzougui et al. (2023) reviewed the most recent advancements in cryopreservation techniques for ornamental flower bulbs, encompassing a range of structures such as bulbs, corms, tubers, tuberous roots, and rhizomes. They reported that distinct cryopreservation techniques were employed across various species, often utilizing diverse explant sources. Although some studies have explored cryopreservation within the Iridaceae family, such as Crocus (Baghdadi et al., 2011), Iris (Shibli, 2000) and Gladiolus (Rajasekharan et al., 1994) using methods like vitrification, slow cooling and encapsulation-dehydration methods, the available information remains notably constrained. More recently, droplet-vitrification methods have gained attention and have been successfully applied to a wide range of plant species. Maślanka and Szewczyk (2021) achieved nearly a 100% regrowth using droplet vitrification on Tulipa tarda, a method successful in other bulbous monocots such as Lilium (Chung et al., 2014), Galanthus (Maślanka et al., 2013) and Narcissus (Maślanka et al., 2016).
The optimization of cryopreservation protocols involves testing different concentrations and exposure times of cryoprotective solutions in each step of cryopreservation. The aim of this study is to find suitable protocols for the cryopreservation of Freesia hybrida using the droplet vitrification method by optimizing the various steps in the process.
Materials and Methods
Plant material
In August, 2021, the National Agrobiodiversity Center in Suwon, Republic of Korea (ROK) received tissue-cultured explants of two freesia cultivars, ‘Sunny Gold’ (IT317798) and ‘Sweet Lemon’ (IT309634), developed at the National Institute of Horticultural & Herbal Science (NIHHS) of the Rural Development Administration (RDA). These explants were grown on Murashige and Skoog (MS) medium (Murashige and Skoog, 1962) supplemented with 1.0 ㎎/L 6-benzylaminopurine (BAP), 0.5 ㎎/L Indolacetic acid (IAA), 30 g/L sucrose, and 8.0 g/L plant agar (Duchefa Biochemie B.V., The Netherlands). The pH of the medium was adjusted to 5.8 before autoclaving at 121℃ for 15 min. Cultures were maintained at 24 ± 1℃ with a 16-h photoperiod under white fluorescent light (50 μmol/m2s). Subculturing occurred every five weeks until an adequate number of uniform shoots were obtained (Fig. 1a). For cryopreservation experiments, uniform shoots were transferred to hormone-free MS medium at the last subculture (Fig. 1b).
Cryopreservation procedures using droplet vitrification
Shoot tips measuring 2 × 2 ㎜ (diameters x length) were obtained from 1-week-old in vitro grown plantlets (Fig. 1c). A standard cryopreservation protocol has been established based on previous studies (Bae et al., 2021, 2022; Lee et al., 2020; Song et al., 2020). The standard protocol consisted of four steps and was conducted under the following conditions. The prepared shoot tips underwent preculturing on MS medium with 0.3 M sucrose for 31 h, followed by 17 h with 0.5 M sucrose. The cultures were kept in the dark at the temperature of 24 ± 1℃ during the incubation period (Fig. 1d). The precultured shoot tips were subjected to osmoprotection using C4 solutions (MS medium supplemented with 17.5% glycerol and 17.5% sucrose) for 40 min. The loaded shoot tips were exposed to 100% PVS3 (B1) dehydration solution for 60 min (Fig. 1e). The shoot tips were transferred onto droplets containing 2.5 μL dehydration solutions and placed on a sterilized aluminum foil strip measuring 4.0 ㎝ × 0.5 ㎝ (Fig. 1f). Subsequently, the shoot tips were directly immersed in LN for a duration of at least one hour for cryopreservation (Fig. 1g).
The cryopreserved shoot tips were removed from LN storage and submerged into unloading solutions warmed in a water bath at 40℃. The room temperature unloading solutions were then replaced two times, each for 15 and 25 min, respectively. The unloading solutions were prepared with MS medium supplemented with 0.8 M sucrose. After thawing, the rewarmed shoot tips were placed onto hormone-free MS medium. The cultures were then incubated under dark conditions at 25 ± 2℃ for two weeks. Subsequently, the cultures were transferred to normal light conditions in the culture room (Fig. 1h, 1i).
To optimize the conditions for each of the four steps in cryopreservation, various treatments were tested alongside the standard protocol in each step. Tested treatments in each step are described below.

Fig. 1.
Cryopreservation of shoot tips of Freesia hybrida ‘Sweet Lemon’ by droplet-vitrification. (a) In vitro grown plantlets of ‘Sweet Lemon’ used for cryopreservation experiments. (b) Shoot tips were cultured on MS medium for a week. (c) Shoot tip is rounded by circle. (d) Dissected shoot tips in preculture solution. (e) Dehydration of shoot tips in vitrification solution (B1) (f) Droplets on sterilized aluminum foil strips. (g) Immersion of aluminum foil along with shoot tips into the liquid nitrogen (LN) for 1 h. (h) Plant regrowth from cryopreserved (+LN) shoot tips 8 weeks after inoculation. (i) A shoot regrowth into whole plant from cryopreserved shoot tips that had been cultured on MS medium.
Preculture
Five different preculture treatments were tested. 1. MS + 0.1 M sucrose for 3 days; 2. MS + 0.3 M sucrose for 3 days; 3. MS + 0.3 M sucrose for 31 h, followed by 0.5 M sucrose for 17 h; 4. MS + 0.3 M sucrose for 55 h, followed by 0.5 M sucrose for 17 h; and 5. MS + 0.1 M sucrose for 1 day, 0.3 M sucrose for 1 day, and 0.5 M sucrose for 1 day.
Loading
Four loading treatments were tested. 1. C4 (MS + 17.5% glycerol + 17.5% sucrose) solution with loading times of 30 min; 2. C4 solution for 40 min; 3. C7 (MS + 18.4% glycerol + 13.75% sucrose) solution with loading times of 20 min; and 4. C7 solution for 40 min.
Dehydration
The compositions of various PVS treatments and their exposure durations are described in Table 1. PVS2 (A variants) treatments were done in the low temperature shaking incubator at 0℃ (Dasol Scientific, Suwon, South Korea) and PVS3 (B variants) treatments were done at room temperature.
Table 1.
Various plant vitrification solutions and exposure durations (Kim et al., 2009)
| PVSz | Total Conc. (%, w/v) | Compositions (%, w/v) | Exposure Times (min) | Remarks |
| A1 | 73.7 | glycerol 30.0 + DMSOy 15 + EGx 15 + sucrose 13.7 | 30 | PVS2 |
| A3 | 90 | glycerol 37.5 + DMSO 15 + EG 15 + sucrose 22.5 | 30, 120 | |
| A7 | 90 | glycerol 37.5 + DMSO 10 + EG 10 + sucrose 32.5 | 30 | |
| B1 | 100 | glycerol 50 + sucrose 50 | 30, 60, 90, 120 | PVS3 |
| B3 | 90 | glycerol 45 + sucrose 45 | 60 | |
| B5 | 80 | glycerol 40 + sucrose 40 | 60 |
Unloading
Three unloading treatments were tested. MS medium supplemented with 0.8 M sucrose for 40 min, 0.8 M sucrose for 60 min, and 1.2 M sucrose for 20 min.
Statistical Analysis
In this study, each treatment consisted of three replicates, with three replicates exposed to LN (+LN) and three replicates not exposed to LN (-LN). Within each treatment, there were ten shoot tips per replication, resulting in a total of thirty shoot tips per treatment. The shoot regrowth rate was assessed as the percentage of direct shoot growth from the shoot tips, noted 8 weeks after thawing. The results are presented as mean values, along with standard errors (SE). The data were analyzed using analysis of variance (ANOVA) and Duncan’s multiple range tests (DMRT), with significance calculated at p < 0.05. Analyses were performed using SAS 7.1 software (SAS Institute Inc., Cary, NC, USA).
Results
Effects of preculture treatments on regrowth rates of non-cryopreserved (-LN) and cryopreserved (+LN) shoot tips from two freesia cultivars
For the ‘Sunny Gold’ cultivar, the regrowth rates of -LN shoot tips reached their peak at 75% after a 3-day period of preculture with 0.3 M sucrose. In contrast, a two-step treatment involving 0.3 M and 0.5 M sucrose concentrations was required to induce shoot regrowth in +LN shoot tips (Table 2). For ‘Sunny Gold’, a treatment sequence involving a 55-hour exposure to 0.3 M sucrose followed by a 17-hour exposure to 0.5 M sucrose yielded a 13% regrowth rate for +LN shoot tips. However, for the ‘Sweet Lemon’ cultivar, only the treatment comprising a 31-hour exposure to 0.3 M sucrose followed by a 17-hour exposure to 0.5 M sucrose led to a 10% shoot regrowth in +LN shoot tips (Table 2). Our results showed that preculture treatments with 0.3 M and a two-step treatment of 0.3 to 0.5 M sucrose significantly increased the regrowth rates of both of –LN and +LN shoot tips.
Table 2.
Effects of preculture treatments on regrowth rates of both non-cryopreserved (-LN) and cryopreserved (+LN) shoot tips from two freesia cultivars ‘Sunny Gold’ and ‘Sweet Lemon’
| Preculture | Regrowth Rates (%)z | |||
| ‘Sunny Gold’ | ‘Sweet Lemon’ | |||
| -LN (%) | +LN (%) | -LN (%) | +LN (%) | |
| 0.1 M 3 d | 15.5 ± 1.20 cy | 0.0 b | 0.0 c | 0.0 b |
| 0.3 M 3 d | 75.0 ± 8.30 a | 0.0 b | 20.0 ± 3.27 abc | 0.0 b |
| 0.3 M 31 h + 0.5 M 17 h | 40.3 ± 5.81 b | 3.6 ± 2.05 b | 27.2 ± 7.42 ab | 10.0 ± 10.00 a |
| 0.3M 55 h + 0.5 M 17 h | 73.7 ± 4.00 a | 13.4 ± 3.35 a | 30.3 ± 5.27 a | 0.0 b |
| 0.1 M 1 d + 0.3 M 1 d + 0.5 M 1 d | 0.0 d | 0.0 b | 8.3 ± 4.17 bc | 0.0 b |
Effects of loading and dehydration treatments on regrowth rates of non-cryopreserved (-LN) and cryopreserved (+LN) shoot tips from two freesia cultivars
The regrowth rates of -LN shoot tips reached their highest levels when treated with the C7 loading solution for a duration of 40 min in ‘Sunny Gold’ and 20 min in ‘Sweet Lemon’. However, shoot regrowth was observed only in the C4 treatments after +LN dipping in both cultivars (Table 3).
Table 3.
Effects of loading treatments on regrowth rates of both non-cryopreserved (-LN) and cryopreserved (+LN) shoot tips from two freesia cultivars ‘Sunny Gold’ and ‘Sweet Lemon’
| Loading | Regrowth Rates (%)z | |||
| ‘Sunny Gold’ | ‘Sweet Lemon’ | |||
| -LN (%) | +LN (%) | -LN (%) | +LN (%) | |
| C4 30 min | 23.3 ± 7.73 by | 7.1 ± 0.00 a | 26.1 ± 3.88 a | 0.0 a |
| C4 40 min | 40.3 ± 5.81 b | 3.6 ± 2.05 ab | 27.2 ± 7.42 a | 10.0 ± 10.00 a |
| C7 20 min | 44.8 ± 11.50 b | 0.0 b | 41.5 ± 11.64 a | 0.0 a |
| C7 40 min | 75.7 ± 4.30 a | 0.0 b | 21.8 ± 5.26 a | 0.0 a |
zPrecultured shoot tips (MS + 0.3 M sucrose 31 h + 0.5 M sucrose 17 h) were treated with varying loading solutions (C4: MS + 17.5% glycerol + 17.5% sucrose; C7: MS + 18.4% glycerol + 13.75% sucrose) and durations, followed by a standard protocol (dehydration: B1 60 min, unloading: 0.8 M sucrose 40 min). The results are presented as mean ± SE.
In terms of optimal conditions for shoot tip dehydration, for ‘Sunny Gold’, the A3 30 min treatment resulted in the highest regrowth rate in -LN shoot tips, but it did not yield shoot regrowth after cryopreservation (Fig. 2). Notably, shoot regrowth of +LN shoot tips was exclusively observed with B1 treatments (Fig. 2). In ‘Sweet Lemon’, shoot regrowth was observed with A3 60 min and B1 for 60, 90 and 120 min treatments in +LN, even though regrowth rates remained low (Fig. 3).
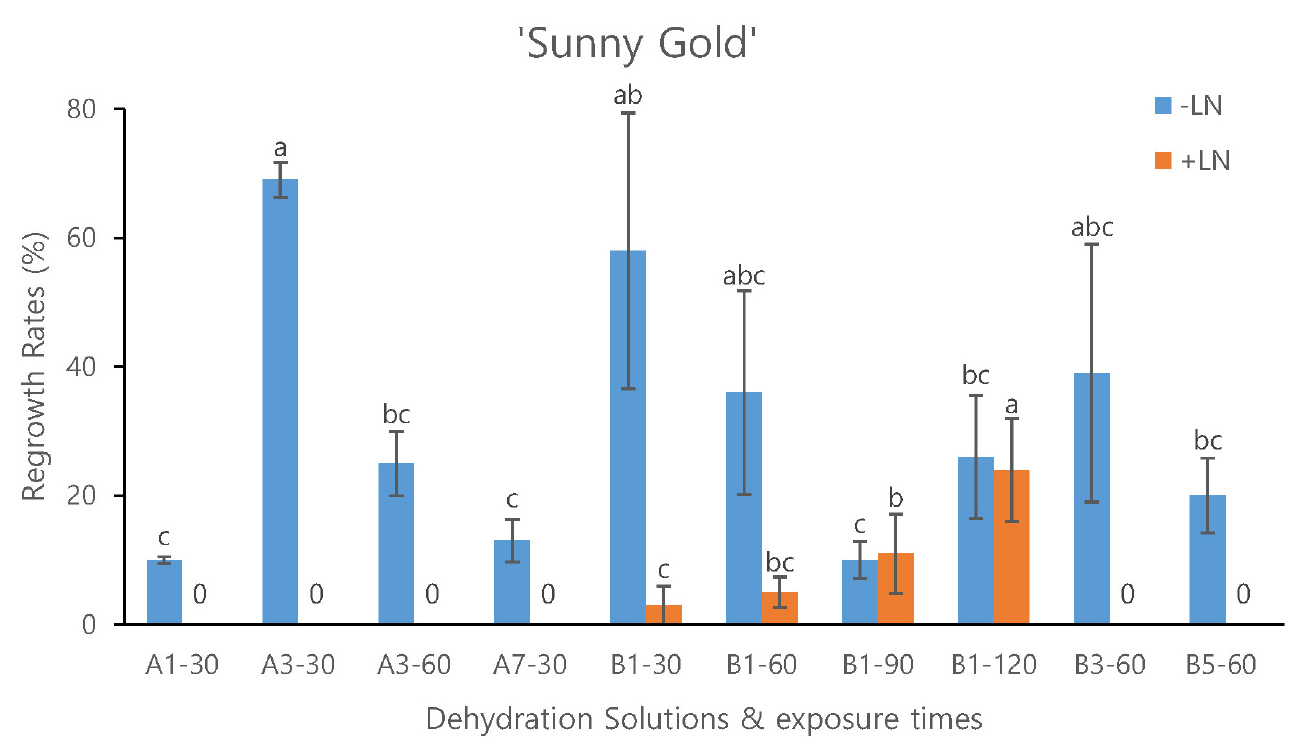
Fig. 2.
Effects of PVS treatments and durations on regrowth rates of both non-cryopreserved (-LN) and cryopreserved (+LN) shoot tips from freesia cultivar ‘Sunny Gold.’ The results are presented as mean values with bars marked by standard errors. Data with different letters are significantly different at p < 0.05.

Fig. 3.
Effects of PVS treatments and durations on regrowth rates of both non-cryopreserved (-LN) and cryopreserved (+LN) shoot tips from freesia cultivar ‘Sweet Lemon.’ The results are presented as mean values with bars marked by standard errors. Data with different letters are significantly different at p < 0.05.
Effects of unloading treatments on regrowth rates of non-cryopreserved (-LN) and cryopreserved (+LN) shoot tips from two freesia cultivars
For the ‘Sunny Gold’ cultivar, regrowth rates among -LN and +LN shoot tips exhibited no discernible difference among unloading treatments (Table 4). Regarding the ‘Sweet Lemon’ cultivar, the 0.8 M sucrose treatment spanning 60 min demonstrated the highest efficacy in promoting regrowth rates among -LN shoot tips. Nevertheless, this treatment did not yield regrowth among +LN shoot tips. Notably, other treatments involving 0.8 M sucrose for 40 min and 1.2 M sucrose for 20 min yielded regrowth rates of approximately 10% (Table 4). It appears that the varying responses in the two freesia can be also attributed to their morphological and physiological differences.
Table 4.
Effects of unloading treatments on regrowth rates of both non-cryopreserved (-LN) and cryopreserved (+LN) shoot tips from two freesia cultivars ‘Sunny Gold’ and ‘Sweet Lemon’
| Unloading | Regrowth Rates (%)z | |||
| ‘Sunny Gold’ | ‘Sweet Lemon’ | |||
| -LN (%) | +LN (%) | -LN (%) | +LN (%) | |
| 0.8M 40 min | 40.3 ± 5.81 ay | 3.6 ± 2.05 a | 27.2 ± 7.42 b | 10.0 ± 10.00 a |
| 0.8M 60 min | 39.6 ± 10.24 a | 6.0 ± 3.47 a | 50.0 ± 5.77 a | 0.0 a |
| 1.2M 20 min | 38.75 ± 1.25 a | 5.5 ± 5.50 a | 13.3 ± 1.93 b | 8.9 ± 4.43 a |
zShoot tips were cryopreserved using a standard protocol (preculture: MS + 0.3 M sucrose for 31 h followed by 0.5 M sucrose for 17 h, loading: C4 for 40 min, dehydration: B1 60 min) and different unloading solutions were applied, consisting of MS medium supplemented with various sucrose concentrations and durations. The results are presented as mean ± SE.
Discussion
The development of a cryopreservation protocol involves understanding the intricate interactions among complex plant tissues and a diverse array of behaviors exhibited under the influence of plant vitrification solutions (PVS). These solutions regulate both osmotic and chemical stresses during the cryopreservation process. Therefore, our study focused on optimizing various sucrose and PVS concentrations, along with exposure durations, at each step of the cryopreservation process. Given the limited available information on cryopreservation techniques for Freesia spp., we have established a standard protocol based on the successes of previous studies and the collective experiences of our laboratory utilizing droplet vitrification methods (Bae et al., 2021, 2022; Lee et al., 2020; Song et al., 2020).
In this study, we examined the effects of different preculture sucrose concentrations and exposure durations on the regrowth rates of both +LN and -LN shoot tips. Two different freesia cultivars, ‘Sunny Gold’ and ‘Sweet Lemon’, induced shoot regrowth with a two-step treatment involving 0.3 M and 0.5 M sucrose concentrations in +LN shoot tips (Table 2). However, different time exposures to 0.3 M sucrose followed by a 17-hour exposure to 0.5 M sucrose were required to produce the highest regrowth rates of +LN shoot tips in two freesia cultivars (Table 2). These results highlight the importance of adjusting the preculture duration based on the specific plant materials, as tissue water content and dehydration tolerance can vary. These findings provide another example of successful regeneration falling within the range of 0.2 M to 0.7 M sucrose, with concentrations around 0.3 M to 0.5 M commonly applied in the cryopreservation of many crops, such as garlic (Liu et al., 2017), strawberry (Bae et al., 2021), potato (Folgado et al., 2015), chrysanthemum (Yi et al., 2018) and more. While some species benefit from prolonged high-sucrose preculture (Chmielarz et al., 2005; Song et al., 2020), our study found no positive effect on shoot regrowth with 0.7 M sucrose or longer durations, up to a week (data not shown).
We also explored the impacts of different loading conditions. Loading treatments can be beneficial in increasing material resistance by using a diluted mixture of PVSs before applying more concentrated and toxic compounds (Kulus and Zalewska, 2014). Optimized loading solutions have been applied to improve the dehydration tolerance of explants in the majority of cryopreservation studies (Kim et al., 2009). While C7 loading solutions have been widely used in many plant materials, our study found that C4 was more effective than C7 treatments, which is consistent with studies on lilium (Song et al., 2020), plum (Vujovi´c et al., 2023) and strawberry (Bae et al., 2021).
Among the critical stages of cryopreservation, the identification of optimal conditions for shoot tip dehydration stands as a paramount endeavor. Previous research has elucidated the constituents and concentrations of PVS (Kim et al. 2009). PVS consists of penetrating and non-penetrating cryoprotectants, which reduce freezable water, allowing cellular vitrification while safeguarding cell membranes during cryopreservation (Mubbarakh et al. 2014). However, it is important to note that PVS are highly toxic. PVS2 induces chemical toxicity containing DMSO and EG, while PVS3 induces osmotic shock. Therefore, the choice of PVS composition must be tailored to the specific plant material (Kulus and Zalewska, 2014). In the case of ‘Sunny Gold’ in this study, a PVS2 variant (A3 30 min treatment at 0℃), resulted in the highest regrowth rate in -LN shoot tips but shoot regrowth in +LN was only observed with PVS3 (B1) treatments (Fig. 2), similar to the study of sweet potato (Park and Kim 2015). This reflects the sensitivity of shoot tips to chemical toxicity and insufficient cryoprotection. These findings align with observations in several plant species, such as garlic (Liu et al., 2017), chrysanthemum (Yi et al., 2018), and Musa (Panis et al., 2005), which reported superior results with PVS3 compared to PVS2. Additionally, we observed that extended exposure to the B1 solution resulted in increased +LN shoot regrowth, but this trend was reversed for -LN shoot tips (Fig. 2). Indeed, finding the optimal conditions presents a challenge, as it involves a delicate balance between preventing intracellular crystallization to enhance cryopreservation under +LN conditions while simultaneously minimizing osmotic shock to cells under –LN conditions. We observed that A3 was effective on ‘Sweet Lemon’ for +LN, which is smaller in size compared to ‘Sunny Gold.’ This observation supports the effectiveness of PVS2 for medium-sized explants and PVS3 for larger-sized explant (Kim et al., 2009; Kulus and Zalewska, 2014; Maślanka et al., 2016). Based on overall results, it appears that ‘Sweet Lemon’ is more sensitive to osmotic shock and dehydration compared to ‘Sunny Gold’.
Rewarming and unloading hold significant importance in cryopreservation processes, serving to prevent recrystallization, and dilute and remove PVS, which can be toxic to plants (Mubbarakh et al., 2014). Our results of varying responses in the two freesia cultivars are consistent with findings of previous studies, highlighting the need to tailor the optimum cryopreservation protocol to specific plant materials (Kim and Popova, 2023).
This study provides valuable insights into freesia cryopreservation methods. Despite the significant impact of CPA treatments on cryopreservation, our results indicate the sensitivity of factors influencing plant tissue survival during cryopreservation. This suggests the need to consider additional factors beyond CPA treatments. In addition, we explored various factors, such as explant size, plant age, incubation conditions, pretreatment and regrowth medium. However, meaningful data from these explorations are yet to be collected. Further studies are warranted to systematically investigate these factors and continually enhance the effectiveness of cryopreservation methods for the long-term preservation of Freesia germplasm.


