Introduction
Materials and Methods
Reagents
Sample preparation
Cell culture and treatment
Cell viability assay
Measurement of NO production
Isolation of cytosol and nucleus fraction
SDS-PAGE and Western blot analysis
Reverse transcriptase-polymerase chain reaction (RT-PCR)
Statistical analysis
Results and Discussion
Effect of GLE on the activation of RAW264.7 cells
Effect of GLE on MAPK/NF-κB signaling pathway activation in RAW264.7 cells
Contribution of p38, JNK and NF-κB to GLE-mediated expression of immunomodulators in RAW264.7 cells
Effect of TLR4 on GLE-mediated expression of immunomodulators through the activation of p38, JNK and NF-κB
Introduction
There are two main types of immune surveillance systems against invading pathogens: the innate immune system and the acquired immune system (Arancibia et al., 2007; Sfondrini et al., 2003). The activation of the innate immune system rapidly regulates the replication of microorganisms and induces the development of adaptive immunity. The acquired immune system identifies specific pathogen peptides presented by antigen presenting cells and activates humoral and cellular immune responses (Akira, 2009).
Macrophages are an important type of innate immune cell which play a critical role in regulating various immunopathological conditions of the inflammatory responses by releasing factors that mediate inflammation (Junt et al., 2007). The activation of macrophages initiates the innate immune response and induces antigen processing and presentation to promote adaptive immunity (Shi and Pamer, 2011). Thus, Macrophages are an important cellular bridge between innate and adaptive immunity. They can not only initiate innate immune responses but also enhance the secretion of cytokines (Zheng et al., 2017). When the body is affected by a variety of stimuli, macrophages secrete of such factors as nitric oxide (NO) and pro-inflammatory cytokines (such as interleukin (IL)-1β, IL-6, IL-12 and tumour necrosis factor (TNF)-α) (Liu et al., 2017). NO is a short-lived inorganic free radical gas derived from L-arginine by NOS activity, which has an antimicrobial effect important in the innate immune system (Boscá et al., 2005; Newstead et al., 2014; Palmer et al., 1988; Park, 2019). Meanwhile, cytokines are key regulators mediating multiple processes, including the activation of T and B cells, anti-tumour processes, and anti-infection processes (Belardelli, 1995; Vitenberga and Pilmane, 2017). NO is mainly produced by inducible nitric oxide synthase (iNOS), and expression of iNOS can be regulated by transcriptional regulators such as nuclear factor kappa B (NF-κB) (Natarajan et al., 2018; Terazawa et al., 2013). Activated NF-κB moves into the nucleus and regulates the expression of pro-inflammatory genes such as iNOS, cyclooxygenase-2(COX-2), and TNF-α (Lee et al., 2017; Ren et al., 2016). In addition, activated mitogen-activated protein kinases (MAPKs) modify the phosphorylation on the threonine and tyrosine motif, accelerating iNOS, COX-2, and pro-infammatory cytokine expression in activated macrophages (Cho et al., 2017; Guo et al., 2015; Hommes et al., 2003; Pearson et al., 2001).
Many epidemiological studies have reported that diets rich in dietary plants significantly reduce the incidence of chronic diseases, infectious diseases, metabolic syndromes, obesity, coronary heart disease, neurodegenerative diseases, and cancer (Giampieri et al., 2014; Gordon et al., 2000; Kesteloot and Zhang, 2000; Lee et al., 2007; Tilman and Clark, 2014). In particular, lettuce (Lactuca sativa L.) is a popular leafy vegetable used in salads or consumed fresh, which are being increasingly consumed as 'healthier' food (Hedges and Lister, 2005). The health-promoting properties of these products are determined by their high levels of antioxidant compounds, primarily vitamin C and polyphenols, as well as fiber (DuPont et al., 2000). They also possess beneficial health effects due to their content in various phytochemicals such as ?avonoids, betalains and carotenoids and the overall high antioxidant capacity such as antioxidant polyphenols as chlorogenic acids and other derivatives of caffeic acid (Crozier et al., 1997; Degl’Innoocenti et al., 2008; Li et al., 2017; Materska et al., 2019; Oh et al., 2009). Caffeic and chlorogenic acids in lettuce have been reported to have various physiological activities such as antibacterial, antioxidant, antitumor and anti-mutagenic (Adesso et al., 2016; El-Seedi et al., 2012; Romani et al., 2002; Wieczyńska and Cavoski, 2018). However, only a few studies have investigated on the mechanism of action of immune-enhanceing activity of lettuce. Therefore, in this study, green lettuce extract (GLE) was prepared and their the immunomodulatory activities and potential mechnism of action of GLE were evaluated in the murine macrophage cell line RAW264.7.
Materials and Methods
Reagents
Chemicals were obtained from Sigma (St. Louis, MO, USA), unless otherwise noted. Cell culture reagents (Dulbecco's Modified Eagle's Medium (DMEM)/F-12, penicillin & streptomycin and fetal bovine serum) were obtained from Lonza (Walkersville, MD, USA). Primers for immune-related gene and housekeeping gene (GAPDH) were obtained from Invitrogen (Grand Island, NY, USA). Primary antibodies against IκB-α, phospho-IκB-α, phospho-IκKαβ, p65, phospho-ERK1/2, phospho-p38, phospho-SAPK/JNK and β-actin were purchased from Cell Signaling (Bervely, MA, USA). The Thermo Scientific Pierce BCA protein assay kit was obtained from Thermo Fisher Scientific (Waltham, MA, USA).
Sample preparation
The green lettuce (Lactuca sativa L.) were purchased from market (Andong, Korea). One gram of freeze-dried green lettuce was extracted with 20 mL of distilled water for 72 h under shaking at the room temperature. After 72 h, the water-soluble extract was filtered and centrifugation at 15,000 rpm for 10 min, the supernatant was freeze-dried to obtain the powder of freeze-dried green lettuce extract (GLE). GLE were dissolved in deionized water and kept in a refrigerator until use.
Cell culture and treatment
RAW264.7 cells as a murine macrophage cell line were purchased from American Type Culture Collection (Manassas, VA, USA) and cultured in DMEM/F-12 media with 10% fatal bovine serum (FBS), 100 U/mL penicillin and 100 ㎍/mL streptomycin and then incubated at 37℃ in 5% CO2 atmosphere.
Cell viability assay
RAW264.7 cells were seeded in 96-well plates. After 24 h, the cells were treated with GLE at various concentration of 0, 25, 50 and 100 ㎍/mL. After 24 h, the cell viability was measured by MTT assay. MTT solution (1 ㎎/mL) was added to each cell, and the cells were incubated for an additional 2 h. After 2 h, DMSO was added to dissolve formazan products, and the plates were shaken for 5 min. The absorbances was recorded on a microplate spectrophotometer at 570 ㎚ using on UV/Visible spectrophotometer (Human Cop., Xma-3000PC, Seoul, Korea).
Measurement of NO production
RWA264.7 cells were seeded in 12-well plate. After 24 h, the cells were treated with GLE at various concentration of 0, 25, 50 and 100 ㎍/mL. After 24 h, NO production was measured by Griess assay. Briefly, 100 μL of the cell culture supernatants were mixed with 100 μL of Griess reagent (Sigma Aldrich, St. Louis, MO, USA) for 15 min at the room temperature. The absorbances was recorded on a microplate spectrophotometer at 570 ㎚ using on UV/Visible spectrophotometer (Human Cop., Xma-3000PC, Seoul, Korea).
Isolation of cytosol and nucleus fraction
Cytosol and nuclear protein from RAW2647 cells were prepared using a nuclear extract kit (Active Motif, Carlsbad, CA, USA) according to the manufacturer's protocols. The cytosol and nuclear protein were retained at -80℃.
SDS-PAGE and Western blot analysis
After treatment, the cells were washed twice with cold 1 × phosphate-buffered saline (PBS), and the cellular proteins were extracted using radioimmunoprecipitation assay (RIPA) buffer (Boston Bio Products, Ashland, MA, USA) supplemented with protease inhibitor cocktail (Sigma-Aldrich) and phosphatase inhibitor cocktail (Sigma-Aldrich). Cell lysates were centrifuged at 15,000 rpm for 10 min at 4℃. The concentration of the proteins extracted from the cells was quantified using bicinchoninic acid (BCA) protein assay (Thermo Fisher Scientific, Waltham, MA, USA). The equal proteins were separated on SDS-PAGE and transferred to PVDF membrane (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The PVDF membranes were blocked for 1 h at room temperature with 5% non-fat dry milk in Tris-buffered saline containing 0.05% Tween 20 (TBS-T) and then incubated with primary antibodies (target proteins) at 4℃ overnight. These blots were washed with TBS-T and incubated with secondary antibodies conjugated to horseradish peroxidase (Santa Cruz, sc-2004). Signals were visualized with an enhanced chemiluminescence kit (Amersham Biosciences, Piscataway, NJ, USA). The membrane was stripped and β-actin signal was detected for the same membrane.
Reverse transcriptase-polymerase chain reaction (RT-PCR)
After treatment, total RNA was prepared using a RNeasy Mini Kit (Qiagen, Valencia, CA, USA) and total RNA (1 ㎍) was reverse-transcribed using a Verso cDNA Kit (Thermo Scientific, Pittsburgh, PA, USA) according to the manufacturer's protocol for cDNA synthesis. PCR was carried out using PCR Master Mix Kit (Promega, Madison, WI, USA) and mouse primers for iNOS, COX-2, IL-1β, IL-6, IL-12, TNF-α, MCP-1 and GAPDH as followed : mouse iNOS: forward 5′-ttgtgcatcgacctaggctggaa-3′ and reverse 5′-gacctttcgcattagcatggaagc-3′, mouse COX-2: forward 5′- gtactggctcatgctggacga-3′ and reverse 5′-caccatacactgccaggt cagcaa-3′, mouse IL-1β: forward 5′-ggcaggcagtatcactcatt-3′ and reverse 5′-cccaaggccacaggtattt-3′, mouse IL-6: forward 5′-gaggataccactcccaacagacc-3′ and reverse 5′-aagtgcatcatcgt tgttcataca-3′, mouse IL-12: forward 5′-aaccagacccgcccaagaac-3′ and reverse 5′-gatcctgagcttgcacgcaga-3′, mouse TNF-α: forward 5′-tggaactggcagaagag gca-3′ and reverse 5′-tgctcctccacttggtggtt-3′ mouse MCP-1: forward 5′-gaaggaatgggtccagacat-3′ and reverse 5′-acgggtca acttcacattca-3′, GAPDH: forward 5′-ggactgtggtcatgagcccttc ca-3′ and reverse 5′-actcacggcaaattcaacggcac-3′. The PCR results were visualized using agarose gel electrophoresis.
Statistical analysis
All the data are show as mean ± SEM (standard error mean). Values were analyzed with SPSS program. One-way analysis of variance (ANOVA) was used to test effects of different GLE extract levels. Once significance was detected, Turkey's honestly significant difference (HSD) test was used for comparing differences between groups as post hc analysis. Significant difference was considered at P < 0.05.
Results and Discussion
Effect of GLE on the activation of RAW264.7 cells
Nitric oxide (NO) produced by the inducible isoform of nitric oxide synthase (iNOS) plays an important role in anti-infectious immune responses as an important modulator in both innate and adaptive immunity (Chen et al., 2010; Lechner et al., 2005; Wen et al., 2016). It is an key messenger molecule in living cells and plays an role in resisting external pathogens (Pautz et al., 2010). Therefore, analysis of the release of NO by activated macrophages can reflect the effects of GLE on immune stimuli, which could be used as a quantitative indicator of macrophage activation. The effects of GLE on NO production in RAW264.7 macrophages were investigated by measuring on the basis of the Griess reaction in the culture medium (Fig. 1A).
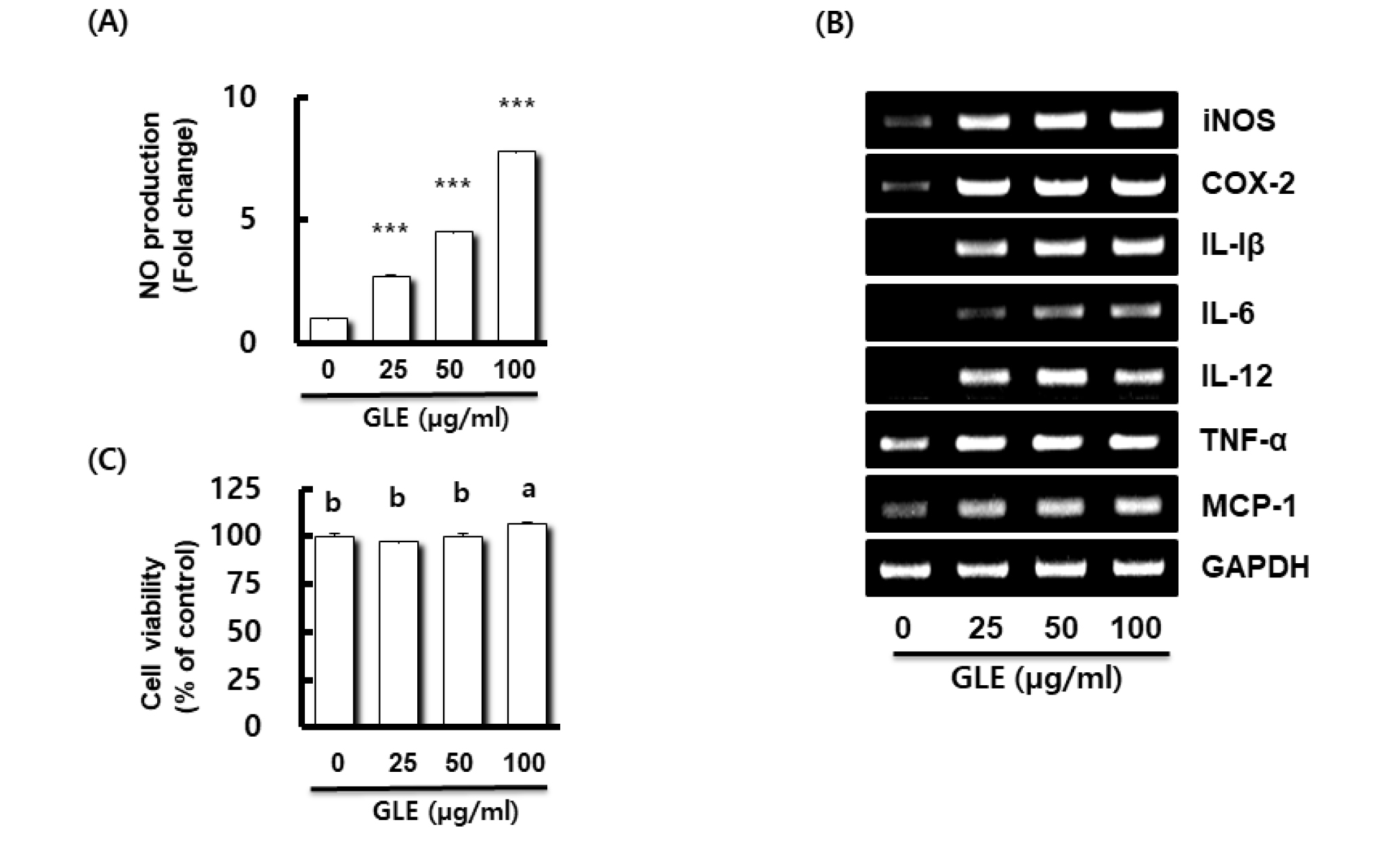
Fig. 1.
Effect of GLE on NO production, and the expression of immune-related gene in RAW264.7 cells. (A) NO production in RAW264.7 cell treated with GLE for 24 h. NO production was measured by Griess assay. Data presented as Mean ± SEM (n=3). ***P < 0.001 compared to the cells without the treatment. (B) Gene expression in RAW264.7 cell treated with GLE for 24 h. For RT-PCR analysis, total RNA was prepared. GAPDH was used as internal control for RT-PCR. (C) Viability in RAW264.7 cell treated with GLE for 24 h. Cell viability was measured using MTT assay. P < 0.05 compared to the cells without the treatment. Data presented as Mean±SEM (n=3). Different letter superscripts mean significantly different sample concentration as analyzed by one-way ANOVA, Tukey test (p < 0.05).
When RAW264.7 cells were treated with various concentrations (25, 50, and 100 ㎍/mL) of GLE, untreated RAW264.7 cells secreted a small amount of NO, and GLE increased the production of NO in a dose-dependent manner at concentrations between 25 and 100 ㎍/mL. Lo et al. (2002) reported that proper NO play useful roles in the antimicrobial activity of macrophages against pathogens. In order to evaluate the immunomodulatory effect of GLE, we investigated whether GLE promotes the expressions of immunomodulators such as iNOS, cyclooxygenase-2 (COX-2), interleukin (IL)-1β, IL-6, IL-12, tumor necrosis factor-α (TNF-α), and monocyte chemoattractant protein-1 (MCP-1) (Fig. 1B). The levels of all immunomodulators increased in response to GLE stimulation. This result shows that GLE significantly increased the production of iNOS, COX-2, IL-1β, IL-6, IL-12, TNF-α, and MCP-1 in RAW264.7 cells. iNOS is the most crucial enzyme to synthesize NO after the activation of macrophages. Our results showed that GLE treatment increased the expression levels of iNOS, which are associated with NO production. GLE was shown to stimulate RAW264.7 cells to release all immunomodulators, which have been implicated as key mediators in the response to immunomodulatory stimuli (Sherwin and Fern, 2005).
Cytokines represent the major factors involved in the communication between T cells, macrophages and other immune cells in the course of an immune response to antigens and infectious agents (Belardelli, 1995). iNOS is the most crucial enzyme to synthesize NO after the activation of macrophages. Cyclooxygenase-2 (COX-2) is an inducible enzyme, catalyzes the transformation of arachidonic acid to eicosanoid, which is a variety of biologically active mediators (Hawkey, 1999; Picot et al., 1994; Suh et al., 2007), during inflammation (Mitchell et al., 1995; Smith et al., 1996; Vane et al., 1994). Interleukin (IL)-1β generated by activated macrophage was known for enhancing the activity of T cell and Natural Killer (NK) cell and B cell maturation. Also, it had the growth inhibition activity of cancer cells. (Garlanda et al., 2013). IL-6 is secreted by T cells and macrophages to stimulate the immune response during infection, was involved in the promote differentiation of B cells, stimulating secretion of antibodies, inducing immunoglobulin (Ig) generation and T cells differentiation (Chen et al., 2001; Delgado et al., 2003). IL-12 is a T helper 1 (Th 1)-type cytokine that activate innate immunity and contribute to the development of type 1 T-helper responses (Chehimi et al., 1994; Lankford and Frucht, 2003; Ma et al., 2004; Trinchieri, 2003). Tumor necrosis factor-α (TNF-α) produced by activated macrophage plays an important role in the resistance of normal hosts to infection and malignant tumor growth, and is the mediator of immune stimulation and inflammatory response. TNF-α regulated activation and growth of T cells by maturing dendritic cells and had destruction of cancer cells, metastasis inhibition of cancer and represents anticancer (Kikuchi et al., 2003; Strieter et al., 1993). Monocyte chemoattractant protein-1 (MCP-1) belongs to the family of CC chemokine and shows potent chemotactic property for monocytes, macrophages, memory T lymphocytes, and natural killer cells (NK) in vitro and represents antitumor (Gu et al., 1999; Singh and Fidler, 1993). The release of these pro-inflammatory cytokines is essential for host survival from infection and is required for the repair of injured tissue (Ohta et al., 2007).
To determine the cytotoxicity of GLE on the RAW264.7 cells was performed the MTT viability assay. RAW264.7 cells were treated with various concentrations (0, 25, 50, 100 ㎍/mL) of GLE for 24 h. As shown in Fig. 1C, when the GLE concentration was treated to 100 ㎍/mL, cell viability was significant increased. These results indicated that GLE was not toxic to RAW264.7 cells. Based on this, subsequent studies were conducted by selecting the treatment concentration of GLE as 50 ㎍/mL.
Recently, Nie et al. (2018) reported that Taigan stem lettuce polysaccharides (SLP), characterized by triple helical chains, content of sulphate esters (Falch et al., 2000; Yu et al., 2017), leading to antioxidant and immunomodulatory activities. According to this study, the SLP have strong immunomodulatory activities as plant derived polysaccharides, resulting in promoting proliferation, phagocytosis and NO production of RAW264.7 cells. These activated macrophages non-specifically ingest and destroy invading pathogens such as bacteria and parasites (Mitchell, et al., 2002). In results above, we suggested that GLE is involved in immunomodulatory activity in RAW264.7 cells.
Effect of GLE on MAPK/NF-κB signaling pathway activation in RAW264.7 cells
The MAPK/NF-κB signaling pathway plays a critically important role in the immune system (Hayden and Ghosh, 2008), which regulate multiple cellular processes, including gene expression, immune response, and cell proliferation (Xi et al., 2011). Activated mitogen-activated protein kinases (MAPKs) signaling pathway is upstream of producing IL-1β, IL-6, iNOS, and pro-inflammatory cytokine in activated macrophages (Cho et al., 2007; Cho et al., 2017; Fisher et al., 2006; Guo et al., 2015; Hommes et al., 2003; Pearson et al., 2001). MAPKs regulate multiple cellular processes, including cell growth, differentiation, proliferation, apoptosis, and response to oxidative stress (Chardin et al., 2017). In this study, we examined the effects of GLE on p-ERK1/2, p-p38 and p-JNK, to assess the possible mechanism by which GLE activates the MAPKs (ERK1/2, p38 and JNK) signaling pathway in RAW264.7 cells. As indicated in Fig. 2A, ERK1/2, p38 and JNK phosphorylation levels were increased when the GLE was treated for 1 h. These results showed that GLE may induce the activation of ERK1/2, p38 and JNK.
NF-κB is an essential transcription factor that regulates genes involved in both the innate and adaptive immune responses. To assess whether GLE regulates NF-κB activation, GLE-mediated phosphorylation levels of IKK and IκBα, and total IκBα were measured in RAW264.7 cells. GLE induced the phosphoylation of IKK and IκBα, and downregulated IκBα protein level, which contributed to p65 nuclear accumulation. These results indicate that GLE may activate NF-κB signaling pathway (Fig. 2A and B). Therefore, GLE exerts its immunostimulatory effects through the activation of the MAPK and NF-κB signaling pathways in RAW264.7 macrophages.
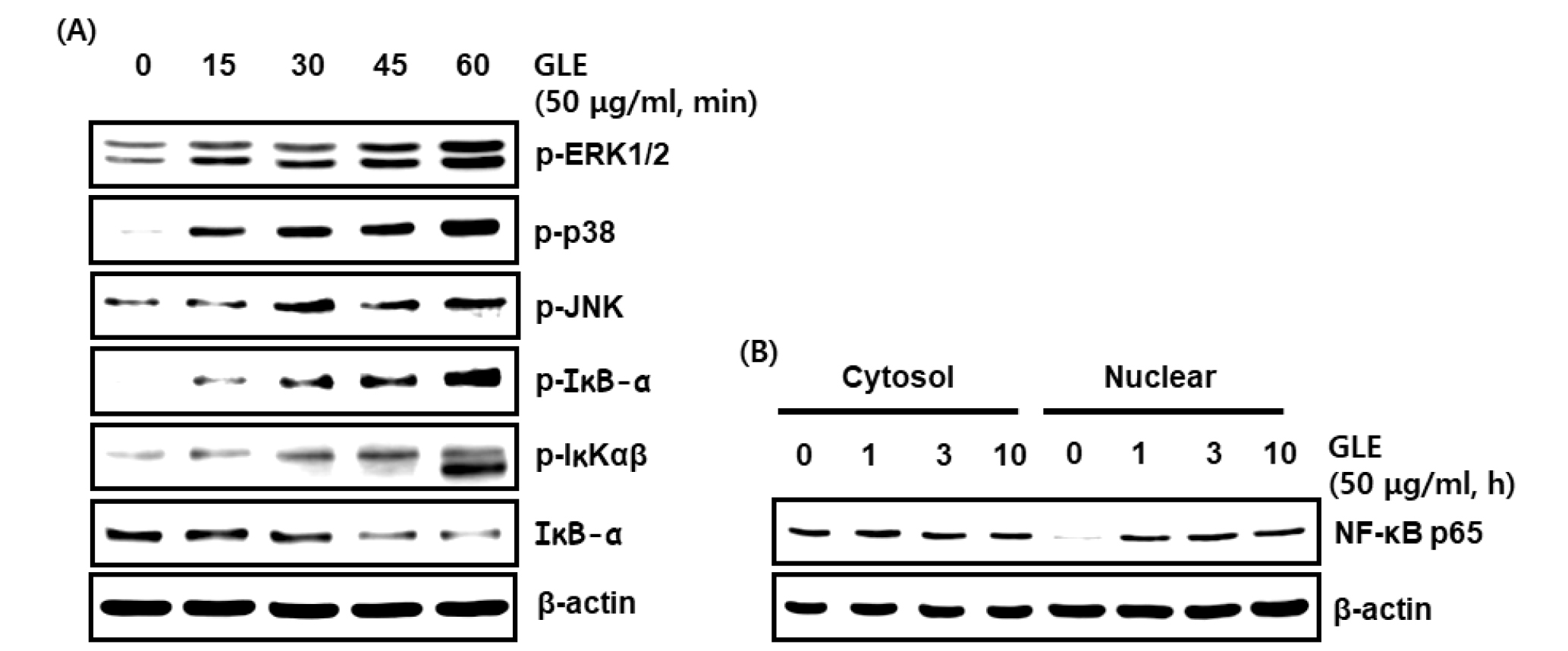
Fig. 2.
Effect GLE on the MAPK/ NF-κB signaling activation in RAW264.7 cells. (A, B) RAW264.7 cells were treated with GLE for the indicated times. All cell lysates were subjected to SDS-PAGE and the Western blot was performed using antibodies against p-ERK1/2, p-p38, p-JNK, p-IκB-α, p-IKK, IκB-α, NF-κB p65 or actin. Actin was used as internal control for Western blot analysis.
Contribution of p38, JNK and NF-κB to GLE-mediated expression of immunomodulators in RAW264.7 cells
The MAPK/NF-κB signaling pathway regulates the production of cytokines related to immune-enhancement (Kopitar-Jerala, 2015). To determine the upstream kinases involved in GLE-mediated cytokine expression, the cells was pretreated with the specific kinase inhibitors such as PD98059 (ERK1/2 inhibitor), SB203580 (p38 inhibitor), SP600125 (JNK inhibitor) and BAY 11-7082 (NF-κB inhibitor), and then co-treated with GLE for 24h. As a result, inhibition of ERK1/2 by PD98059 did not affect GLE-induced expression of IL-1β and IL-6 (Fig. 3A). However, inbition of p38 by SB203580, JNK by SP600125 and NF-κB by BAY 11-7082 blocked the expression of IL-1β and IL-6 (Fig. 3B~D). These results indicate that GLE-mediated expression of immunomodulators may be dependent on p38, JNK and NF-κB.
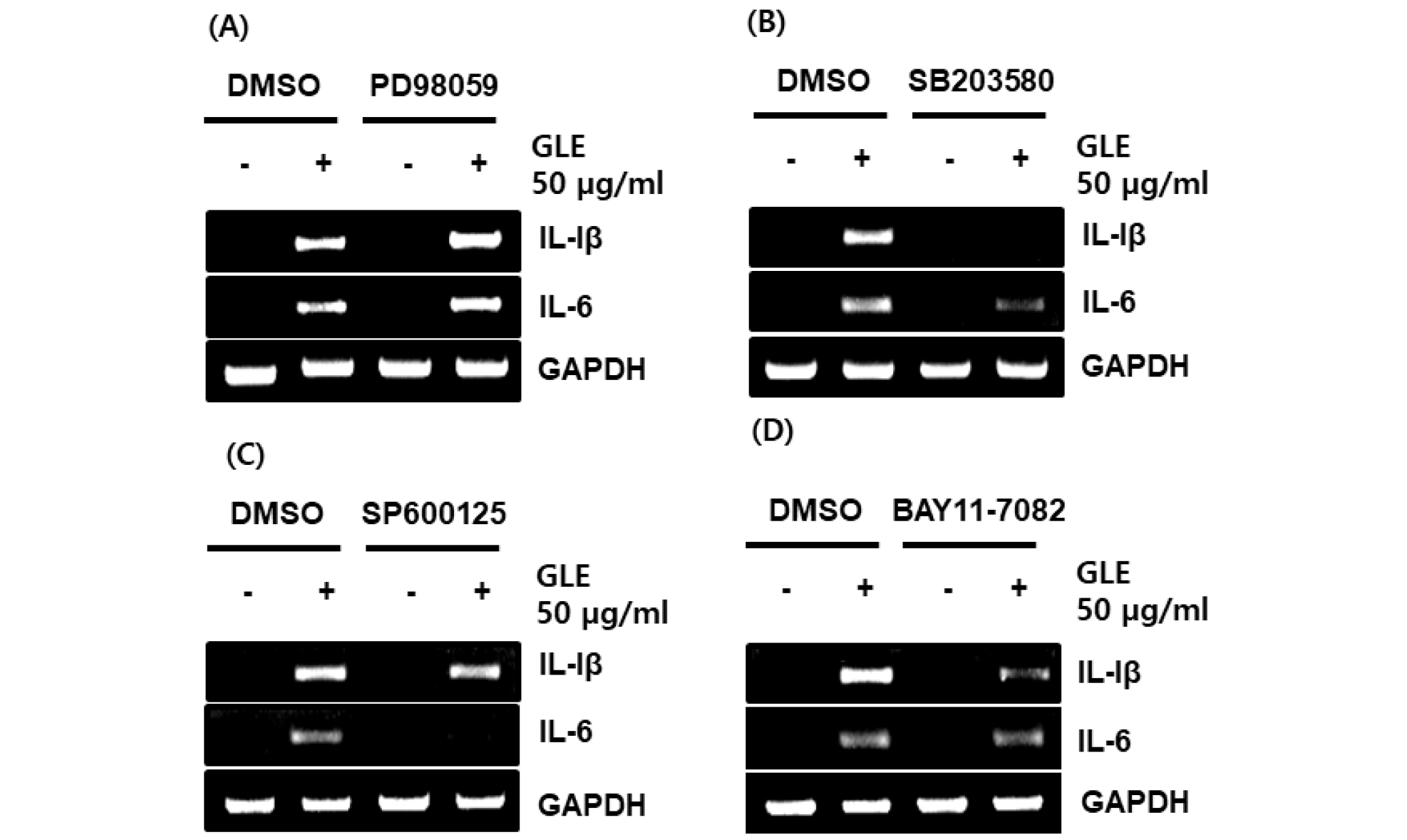
Fig. 3.
Effect of MAPK and NF-κB signaling on GLE-mediated expression of immune-related genes such as IL-1β and IL-6 in RAW264.7 cells. The cells were pretreated with PD98059 (20 μM), SB203580 (20 μM), SP600125 (20 μM) or BAY 11-7082 (20 μM) for 2 h and then co-treated with GLE (50 ㎍/mL) for 24 h. For RT-PCR analysis, total RNA was prepared. GAPDH was used as internal control for RT-PCR.
Effect of TLR4 on GLE-mediated expression of immunomodulators through the activation of p38, JNK and NF-κB
Innate immune surveillance system is a first line defense mechanism against invading microorganisms (Akira, 2009). The activation of the innate immune response rapidly regulates the replication of microorganisms and induces the development of adaptive immunity. Innate immunity differentiates self and non-self by a limited number of germline-encoded receptors, and previously considered as a non-specifc defense against invading microorganisms (Sfondrini et al., 2003). But recent studies have shown that this response depends on so-called pattern-recognition receptors (PRRs) to elicit a defensive response against a vast array of pathogens. These receptors are called pattern recognition receptors (PRRs), as they recognize molecular patterns of components specifc to microorganisms such as carbohydrates, lipids, proteins, lipoproteins and nucleic acids (Akira, 2009; Labonte et al., 2014; Pluddemann et al., 2011). Immunomodulators stimulate the immune system through pattern recognition receptors (PRRs).
Toll-like receptors (TLRs) are one of the best studied the pattern recognition receptor (PRR) families. The recognition of microbial pathogens and their components by TLRs triggers the activation of intracellular signaling and results in production of inflammatory mediators such as nitric oxide (NO), prostaglandins, cytokines such as interleukin (IL)-1β, IL-6, and tumor necrosisfactor (TNF)-α and chemokines (Kawamoto et al., 2008). TLR4 is a membrane-bound toll-like receptor family which is a kinds of the pattern recognition receptors (PRRs), recognises the gram-negative product lipopolysaccharide (LPS) (Brubaker et al., 2015; Kawai and Akira, 2010; O'Neill et al., 2013). The activation of macrophage-mediated immune responses by polysaccharides activates downstream MAPK, NF-κB and other pathway proteins via the TLR4 receptor (Lei et al., 2015; Zhang et al., 2017). TLR4 induces innate immune response by transcriptional regulation, which leads to the production of immunomodulators (Drummond and Brown, 2011; Deretic et al., 2013; Lamkanfi and Dixit, 2014). Thus, we evaluated the effect of TLR4 on GLE-mediated expression of immumodulators. As shown in Fig. 4A, TLR4 inhibition by TAK-242 suppressed the expression IL-1β and IL-6 by GLE in RAW264.7 cells. In addition, we found that TLR4 inhibition by TAK-242 attenuated the phosphoylation level of p38, JNK and IκB-α. These results indicate that TLR4 may be involved in GLE-mediated expression of immunomodulators through the activation of p38, JNK and NF-κB.
In conclusion, considering the present results, it is thought that GLE activates p38, JNK and NF-κB through stimulation of TLR4 and continuously increases the production of immunomodulators in macrophage, which is thought that GLE can contribute to enhancing the immune function of the human body through macrophage activation. GLE may be considered to have immune-enhancing activity and expected to be used as a potential immune-enhancing agent.




