Introduction
Material and methods
Extraction and solvent fractionation
Total phenolic contents
ABTS radical scavenging activity
Cell culture
Anti-inflammatory activity
Anti-cancer activity
Metabolite analysis
Statistical analysis
Results
Total phenolic contents
Anti-oxidant activity
Anti-inflammatory activity
Anti-cancer activity
Chemical profiling of RGM
Discussion
Introduction
Ginseng (Panax ginseng) has been used as an invaluable medicinal herb in oriental medicine (Lee et al., 2015b). In Korea, ginseng is usually classified into white ginseng (4-6 years old and dried after peeling), fresh ginseng (less than 4 years old) and red ginseng (6 years old, steaming and drying process treated) (Yun, 2001). Among these ginseng, red ginseng is the most popular health functional food, accounting for most of the total functional food market (Baeg and So, 2013). Polysaccharide, polyacetylenes, phenolic compounds, and ginsenosides have been reported in ginseng (Attele et al., 1999; Choi, 2008; Jin et al., 2012; Lee et al., 2010). Diverse constituents of ginseng showed numerous biological and pharmacological activities such as anti-oxidant, anti-cancer, anti-inflammatory, anti-fatigue, anti-aging, cytokine regulation, and neurodegenerative diseases (Cho, 2012; Wang et al., 2016; Han et al., 1979; Kim et al., 2019; Lim et al., 2015; Parket al., 2010; Seo et al., 2020; Shin et al., 2000; Sohn et al., 2012; Song et al., 2004, 2003; Sun, 2011; Wang et al., 2014).
To extract these functional compounds of red ginseng, hot water and ethanol have been used for extraction solvent and extracts were formulated as a drink. However, the existing extract process does not effectively extract the bio-active compounds (Lee et al., 2016). Red ginseng marc (RGM), a residue from this process, remains, which contains non-polar substances. A large amount of RGM remains during the process of manufacturing functional foods using red ginseng (Jung et al., 2015). Although RGM is a by-product, recent researches have reported anti-oxidant, anti-cancer and anti-inflammatory activity (Bak et al., 2012; Lee et al., 2015a, 2009) and active compounds such as polysaccharides and non-polar components (Bak et al., 2012; Yoo et al., 2009). However, despite the functionality of the RGM, it is used on low-valued products such as fertilizer or forage. Also, previous researches used limited methods such as hexane extraction. Therefore, to promote the value of RGM, various biological activities of the extracts and fractions of RGM were examined and the methods to extract effectively the non-polar active compounds of RGM were compared.
Material and methods
Extraction and solvent fractionation
Dried RGM was obtained from Korea Tobacco & Ginseng (KT&G) corporation. RGM power was extracted with 100% methanol or hexane at 60℃ for 2 hours using the reflux extractor. Whatman No. 2 filter paper (Whatman International Limited, Kent, England) was used filtering out the impurities of RGM extract. This RGM extract was evaporated under decompression at 60℃ using a rotary evaporator (EYELA, JP/NE-2001, Tokyo, Japan). After that the methanol extract of RGM (RGMM) was suspended in distilled water and fractionated with hexane (RGMMH), ethyl acetate (RGMME), butyl alcohol (RGMMB) and water (RGMMW), progressively.
Total phenolic contents
To evaluate total phenolic contents (TPC), Folin-Ciocalteus’s phenol reagent method (Singleton and Rossi, 1965) was used with some modification. 10 μL of each sample was added into 96 well plate and mixed with 2% Na2CO3. After 3 minutes, 1 N Folin-Ciocalteus’s phenol reagent was added and reacted with the mixture for 27 minutes. The absorbance of each sample was measured at 750 ㎚ using a UV/Vis spectrophotometer (BioTek, Power Wave XS, USA). To evaluate total phenolic contents, gallic acid (Sigma-Aldrich, St. Louis, MO, USA) was used as a standard compound. The results of measurement were calculated using the calibration curve of gallic acid and expressed as g gallic acid equivalent per 100 g (g GAE/100 g).
ABTS radical scavenging activity
ABTS [2,2-Azinobis-(3-ethylbenzthianoline-6-sulphonate)] decolorization method (Re et al., 1999) was performed with some modification. For the ABTS solution used in the experiment, 2.45 mM potassium persulfate solution and 7 mM of ABTS stock solution were reacted for 12 hours in the dark to generate radicals. After then, ABTS solution was diluted with ethanol. In 96-well plate, ABTS solution reacted RGM samples. The mixture was determined at 734 ㎚ using a UV/Vis spectrophotometer. All results were calculated by comparing the variation of absorbance relative to control group.
Cell culture
Raw 264.7 (mouse musculus macrophage) and human HepG2 (human hepatocellular liver carcinoma) cell lines were purchased from Korea Cell Line Bank (Korean Cell Line Research Foundation, Seoul, Korea). Cell lines were cultured Dulbecco’s modified Eagle medium (DMEM high glucose; Hyclone, Logan, UT, USA) containing 10% heat-inactivated fetal bovine serum (FBS; Hyclone, Utah, USA) and 1% penicillin-streptomycin (100 U/mL penicillin and 100 ㎍/mL streptomycin in 0.85% NaCl; Invitrogen, USA). The cell incubator was maintained at humidified 37℃ with 5% CO2.
Anti-inflammatory activity
Anti-inflammatory assay was conducted by modifying Marcocci method (Marcocci et al., 1994). Briefly, the amount of nitric oxide (NO) formed in the lipopolysaccharide (LPS) induced Raw 264.7 mouse macrophage cell with RGM sample was calculated relative to the LPS untreated group. Raw 264.7 cells (1 × 105 density) were seeded in 96-well plate and stabilized. After 20 hours, LPS was treated to induce inflammatory and each sample was added in well. To evaluate NO level, griess reagent A (2% sulfanilamide in distilled water; Promega, Madison, WI, USA) was reacted for 15 minutes. And then, griess reagent B (0.2% naphthyletylene-diaminedihydro-chloride in 4% phosphoric acid; Promega) was added in each well. The absorbance of mixture was read by UV/Vis spectrophotometer at 550 ㎚. The results of NO level were calculated relative to the LPS-nontreated group. The cell viability of RGM samples on Raw 264.7 cells was examined using following MTT assay.
Anti-cancer activity
To evaluate anti-cancer activity of RGM, MTT assay was performed with HepG2 cell, a human-derived liver cancer cell line. In brief, cancer cells were cultured in 96-well plate at a density of 1 × 105 cells per well and incubated. After 24 hours of incubation, samples of different concentrations were added to each well. To perform MTT assay, 0.5 ㎎/mL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT; Sigma-Aldrich) solution was added to the each well and reacted with mitochondrial reductase enzyme. After DMSO treatment, the absorbance was detected at 540 ㎚ using a UV/Vis spectrophotometer. The anti-cancer activities of samples were evaluated by comparison with the untreated group.
Metabolite analysis
In order to analysis the metabolites of the extracts and fractions of RGM, HPLC analysis was performed. Each RGM sample was analyzed by Shimadzu LC-20AD system (Shimadzu corporation, Kyoto, Japan), connected with a SIL-20A auto sampler, a CBM-20A system controller, a LC-20AD solvent delivery module pump, a CTO-20A column thermostat, a SPD-M20A photo diode array UV-Vis detector and YMC-Triart C18 column (250 × 4.6 ㎜ LD, S-5 ㎛, 12 ㎛, YMC Co. Ltd, Kyoto, Japan). All samples were eluted with 0.5% phosphoric acid in distilled water (A) and 0.5% phosphoric acid in acetonitrile (B) with flow rate of 1.0 mL/min at oven temperature 45℃. The gradient elution was used as follows: 0 min 30% B; 0-10 min, 60% B; 10-15 min, 70% B; 15-25 min, 80% B; 25-35 min, 90% B; 35-45 min 100% B; 45-53 min 100% B; 53-55 min 60% B; 55-60 min, 30% B. Analysis was performed at 230 ㎚ using a photo diode array (PDA) detector.
Statistical analysis
All measurements were conducted at least 3 times and the data were expressed as mean ± standard deviation values. The results were compared using an analysis of variance followed by Sutdent’s t tests. P < 0.05 was considered significant.
Results
Total phenolic contents
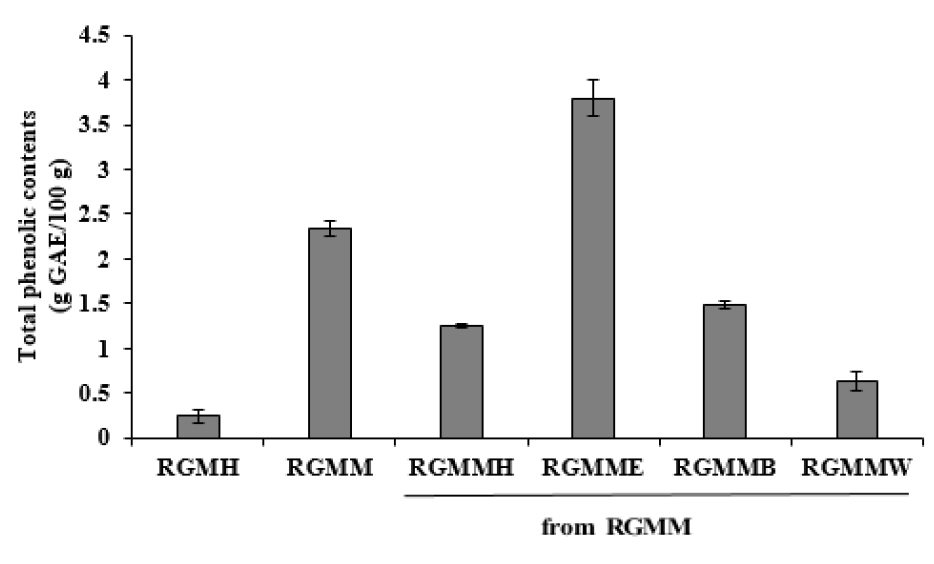
The Folin–Ciocalteu’s method was used to evaluate the total polyphenol contents (TPC) of RGM. The TPC of the ethyl acetate fraction of RGM (RGMME) (3.79 ± 0.21 g gallic acid equivalent (GAE)/100 g) was higher than the methanol extract of RGM (RGMM) (2.35 ± 0.08 g GAE/100 g). The hexane fraction of RGM (RGMMH) (1.25 ± 0.02 g GAE /100 g) and the butanol fraction of RGM (RGMMB) (1.48 ± 0.04 g GAE/100 g) had low TPC (Fig. 1). As a result of TPC assay, each fraction from RGMM showed a distinct difference in phenolic contents.
Anti-oxidant activity
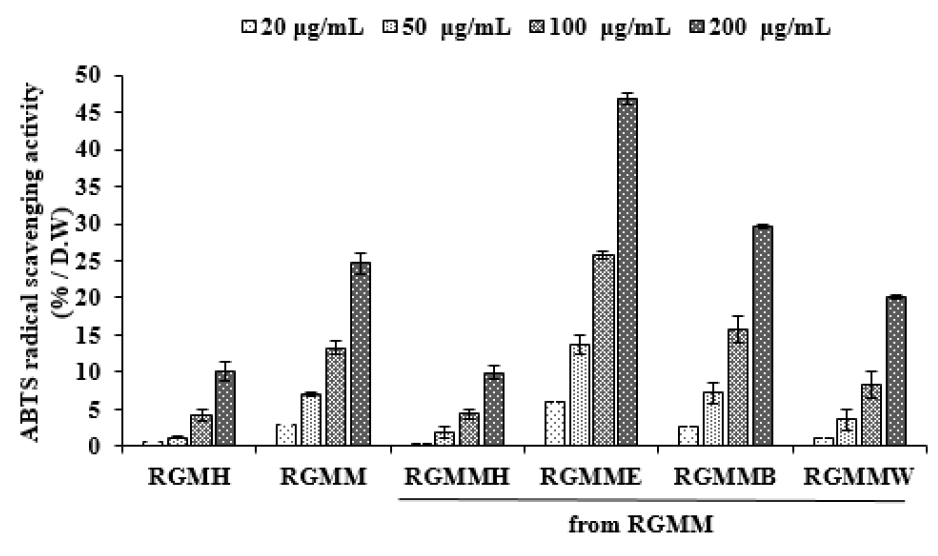
ABTS reagent is blue radical substance. When ABTS radical reacted with hydrogen donor, the color of reagent changes from blue to colorless. As shown in Fig. 2, as the concentrations of the RGMM and each fraction increased, ABTS radical scavenging activity increased. The highest activity was observed in the RGMME (48.86 ± 0.85% at 200 ㎍/mL) comparing with RGMMH (24.62 ± 1.31% at 200 ㎍/mL) and RGMMB (39.66 ± 0.26% at 200 ㎍/mL). Based on the results of the experiments, anti-oxidant activity of RGM is related on TPC, and it was supposed that the antioxidants of RGM were phenolic compounds.
Anti-inflammatory activity
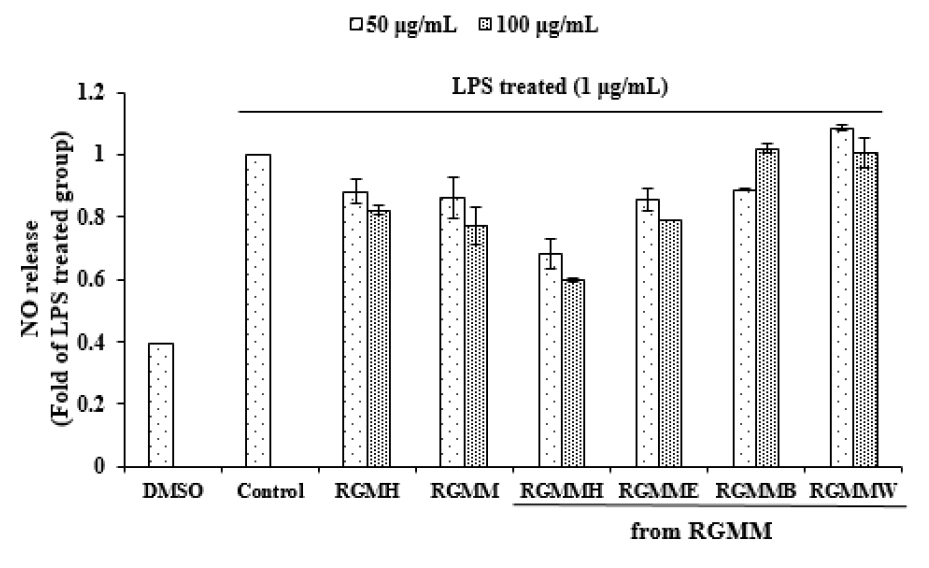
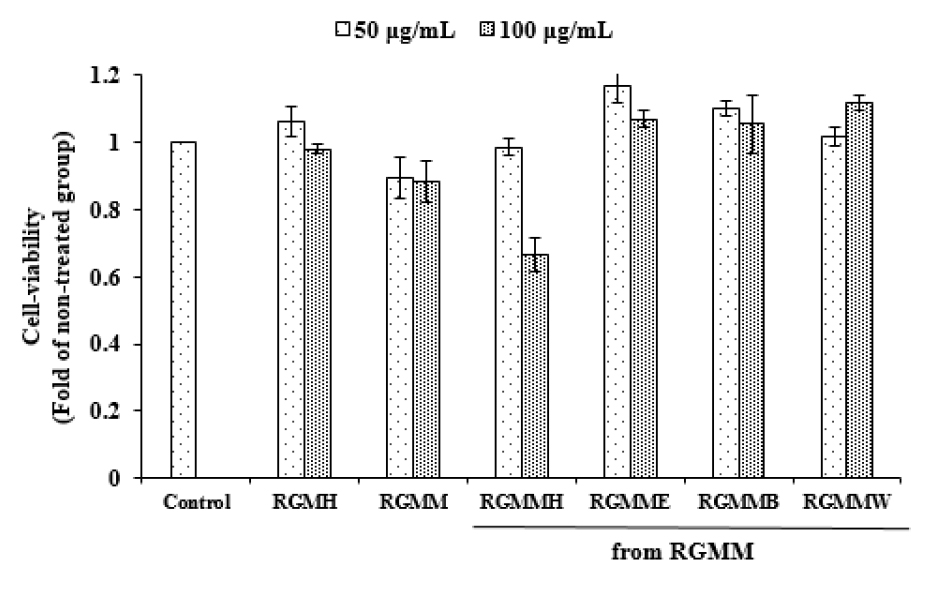
NO possesses cytotoxic properties for pathogenic microorganisms that invade the our body, but if it is excessively produced, it can damage body tissue and causes inflammation (Korhonen et al., 2005). To analyze the anti-inflammatory activities of various fractions from RGM, the inhibition on NO production was evaluated (Fig. 3). Each RGM sample was treated in Raw 264.7 macrophage cells. RGMMH (65.72 ± 1.33% at 100 ㎍/mL) showed distinct anti-inflammatory activity. Meanwhile, RGMH showed weak anti-inflammatory activity in Raw 264.7 cells. The cell viability of RGM samples on Raw 264.7 cells was examined. As shown in Fig. 4, various fractions of RGMM did not influence on Raw 264.7 cell viability remarkably, except RGMMH.
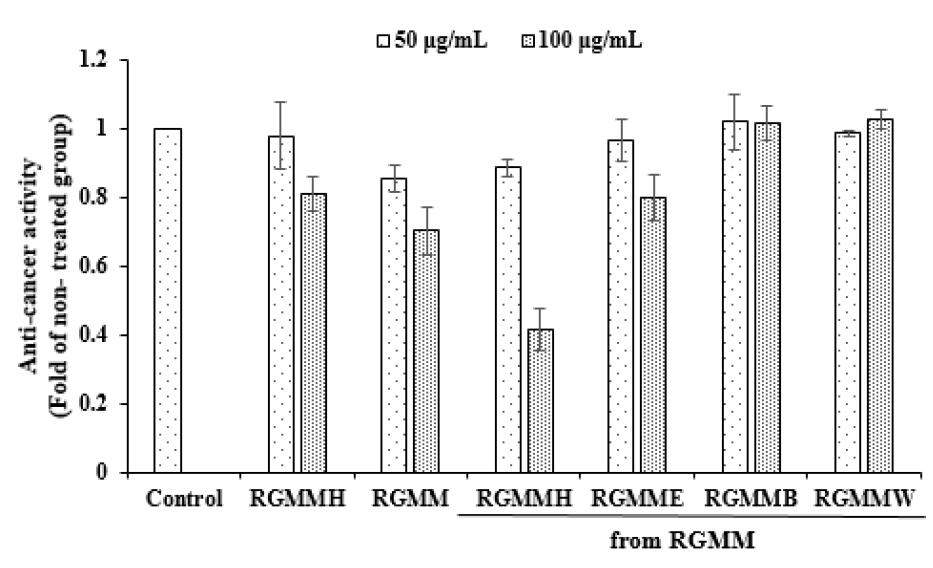
Anti-cancer activity
MTT assay is dependent on the metabolism of dehydrogenase present in mitochondria in living cells (Twentyman and Luscombe, 1987). Dehydrogenase oxidizes the water-soluble yellow formazan into the water-insoluble violet formazan.
To evaluate the anti-cancer activity of RGM, each fraction was treated with HepG2 human cancer cells (Fig. 5). RGMMH showed distinct anti-cancer activity with the potential of 58.56 ± 6.04% at 100 ㎍/mL. RGMME showed anti-cancer activity of 20.13 ± 6.8% at 100 ㎍/mL. RGMMH showed very low TPC, which suggests that the anti-cancer and anti-inflammatory activities of RGMMH are not related to phenolic compounds.
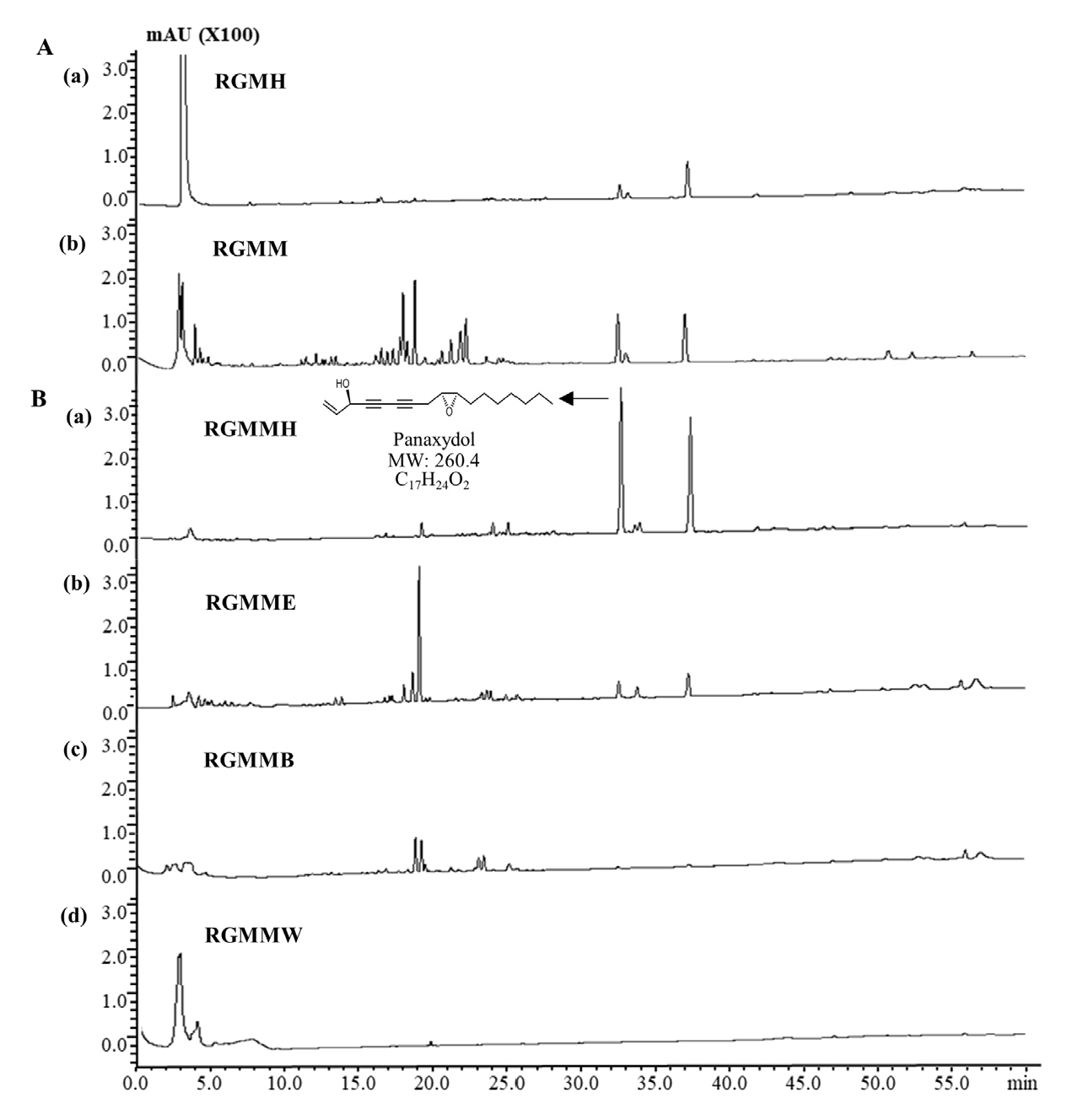
Chemical profiling of RGM
Red ginseng is well known for various active constituents including ginsenoside. Hot water extract of red ginseng has been mainly used for its commercial use, but it does not extract non-polar active compounds effectively. Also, when heat and pressure are applied, the biological activity of RGM is changed and the structures of various constituents transformed. Various extracts and fractions of RGM showed anti-cancer, anti-inflammatory activities (Table 1). To elucidate anti-cancer and anti-inflammatory components of RGM, the metabolites of RGM were examined by HPLC analysis. Chemical profiling of RGM shows that RGMMH have two main peaks at the retention time (RT) 32.6 min and 37.27 min. The peak at 32.6 min was identified as panaxydol by comparison study with the standard. Panaxydol content in RGMMH is 4.5 times higher than in RGMH and many compounds were found in trace amount in RGMMH (Fig. 6). Additional study will be needed to elucidate active compounds of RGMMH.
Table 1.
Biological activities and total phenolic contents of RGM extract and fractions
Discussion
In oriental medicine, red ginseng has been considered as a panacea. In modern times, numerous researches have demonstrated its effects on various diseases (Lee et al., 2015b). RGM is a by-product of red ginseng and it has various biological activities (Bak et al., 2012). But it has been largely discarded. To increase the usage of RGM, the biological activity of RGM was examined. RGMME has anti- inflammatory activity (38.7 ± 1.6% at 100 ㎍/mL) and ABTS radical scavenging activity (48.9 ± 0.85% at 200 ㎍/mL) and high phenolic contents (3.8 ± 0.2 g GAE/100 g). RGMMH also showed potent anti-cancer activity (58.6 ± 6.0% at 100 ㎍/mL) and anti-inflammatory (65.7 ± 1.3% at 100 ㎍/mL). In order to elucidate the non-polar bioactive components of RGM, RGM oil extract was prepared with hexane and its biological activities were examined. Unexpectedly, RGM oil extract (RGMH) showed very weak anti-cancer (16.4 ± 3.3% at 100 ㎍/mL) and anti-inflammatory (29.5 ± 2.1 % at 100 ㎍/mL) effects. Meanwhile, the result of chemical profiling showed that the amounts of main components of RGMMH is higher than RGMH. Among major compounds, panaxydol content is 4.5 times higher in RGMMH than in RGMH. Other compounds were also found in trace amount in RGMMH. These results suggest that non-polar bio-active components of RGM can be extracted effectively by hexane fractionation of the methanol extract.