Introduction
Materials and Methods
Materials and Methods
Analysis of useful substances
Bioactivity measurements
LC-mass spectrometry (LC-MS) apparatus and operating conditions for quantification
Statistical analysis
Results
Discussion
Introduction
Common Fagopyrum esculentum (F. esculentum, Buckwheat), a member of Polygonaceae, is cultivated worldwide and its grains are used for preparing various food products, such as bread, noodles, and flour; the plant is also used for fodder and in medicinal preparations, among other uses.
F. esculentum is an alternative to some of the major food crops in the world (Isérin et al., 2001; Kim et al., 2000; Kim et al., 2007; Kreft et al., 1994; Park et al., 2005), as it contains 60~70% carbohydrate, and approximately 13% protein, essential amino acids, and minerals (Choe et al., 1991; Lee et al., 1991; Lee and Sohn, 1992). Additionally, F. esculentum has a large amount of biologically active material, much of which comprises phenolic compounds. Representative phenolic substances include gallic acid, catechin, quercetin, and rutin, among others. Commonly found in plant leaves, roots, fruits and seeds, these compounds have been shown to act as natural antioxidants with scavenging activity on free radicals and reactive oxygen species (Halliwell et al., 1992; Kim et al., 2005; Pratt, 1992).
Although commonly many useful substances are readily extracted using any of a variety of organic solvents, these organic solvents may generate toxic substances that cause disease, whereby their use has become increasingly restricted for extracting natural substances with biological activity (Chemat et al., 2012; Kimura et al., 1971; Tephly, 1991).
On the other hand, although water extraction is quite innocuous, the extraction yield of most phenolic compounds in water is low (Holasova et al., 2002; Watanabe et al., 1997). Therefore, various methods for increasing the solubility of insoluble phenolic compounds in water have been studied. Microwave technology reportedly increases the solubility of phenolic compounds in water and has been used for analysis for decades now(Kingston and Jassie, 1988). Microwaving causes penetration of light energy into the organic material and generates a vibration within cell walls, which can be converted to a heating effect. The water in the plant matrix destroys the cells by internal overheating and promotes the detachment of useful substances from the matrix. This is the mechanism whereby microwaves improve the extraction yield of useful substances from plant tissue homogenates (Labbozzetta et al., 2005; Rodríguez-Rojo et al., 2012; Vian et al., 2011).
Microwave research for extraction of active compounds present in F. esculentum has already included solvent mixtures (alcohol and water). However, to our knowledge, no studies on water-insoluble phenolic compounds (e.g., rutin, quercetin, catechin) have been reported. The present study was performed to determine the effects of microwave assisted water extraction (MAE, MW) on solubility of low solubility phenolic compounds and their bioactivity. The extracted compounds were identified by LC-MS.
Materials and Methods
Materials and Methods
F. esculentum used in the experiment was produced in Iksan (July, Jeonbuk, South Korea), and used after grinding. The reagents were purchased from Sigma Chemical Co. (St. Louis, MO, USA). F. esculentum was quantified at 10% ratio, and distilled water extraction was conducted at 20, 40, 60, 80, 90℃, or by microwave (700 W) treatment. Ethanol (EtOH) and methanol (MeOH) extractions were performed at 20, 40, 60, and 70℃. Extraction time was one to five minutes at one minute intervals. For subsequent analysis, impurities were removed from the extracts by filtration (Membrane filter, size 0.45 ㎜, Hyundai micro, Korea).
Analysis of useful substances
Phenol and flavonoid contents were determined according to methods reported by Dewanto et al. (Dewanto et al., 2002). The calibration curve was prepared using gallic acid (phenol content) and catechin (flavonoid content).
Bioactivity measurements
Antioxidants were measured by DPPH (2,2-diphenyl-1-picrylhydrazyl) scavenging using the method by Blois (Blois, 1958) and ABTS (2,2'-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid)) scavenging was measured by the method of Kang (Kang, 2014) for free radical scavenging activity. Free-radical scavenging activity was each extraction solvent as the control group, is represented by the scavenging rate (%) = (1- (absorbance sample / absorbance control)) × 100. Absorbance values of the solution were measured using a UV / Vis-spectrometer (UV-1601, Shimadaz, Japan).
LC-mass spectrometry (LC-MS) apparatus and operating conditions for quantification
Quantitative analysis of the substances under study was performed using an Agilent 6410B Triple Quadrupole LC-MS (Agilent Technologies, Wilmington, USA). For each sample, 100 ㎎ of tissue sample and 900 µL of solvent (methanol) were mixed and centrifuged. LC-MS was carried out, with details shown in Table 1.
Table 1. LC-MS method table
Statistical analysis
The experiments were performed three times with three replicates each time (n=3). Data are means ± standard deviation.
Results
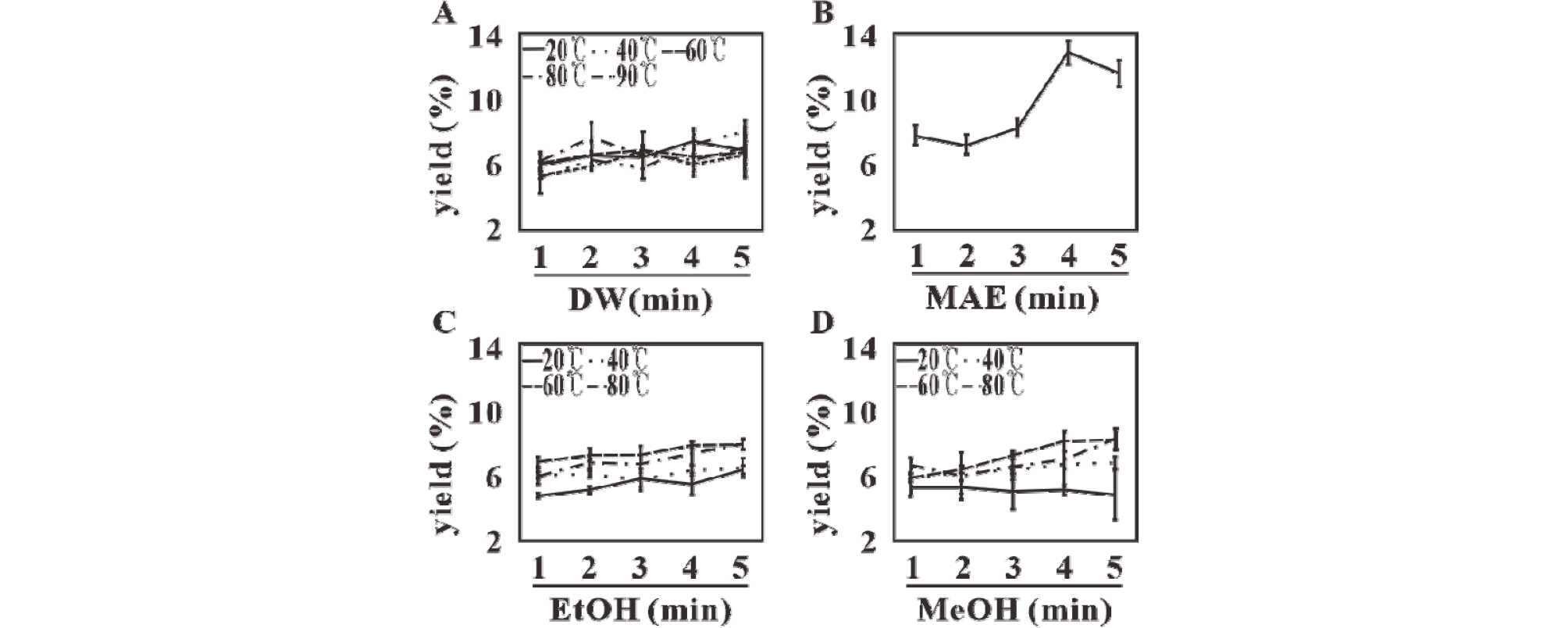
Extraction yield measured by the dry weight of the extract is shown in Fig. 1. Yield by distilled water extraction (DWE) increased with increasing temperature and time, although it was lower than the yield obtained with other solvents and electromagnetic extraction. Although the yield obtained using alcoholic extraction at room temperature (RTE) appeared to be lesser than the yield of distilled water extraction, high temperature extraction (HTE) yielded more than DWE.
However, MAE yield was higher than with alcohol extraction and increased up to four minutes but decreased after five minutes (Table 2).
Table 2. Confirmation of the yield of the extract according to the conditions of F. esculentum
Since internal moisture decreased, homogenates subjected to MAE turned gelatinous at 90℃ in two to three minutes, and the solute at the bottom began to solidify after treatment for five minutes. Bioactivity analysis of useful substances was based on the determination of phenol and flavonoid content, DPPH and ABTS scavenging ability (Table 3). The bioactivity of useful substances in all solvents increased with temperature and time. The bioactivity of compounds extracted by DWE or RTE was similar to the bioactivity of the same compounds extracted using alcohols. Furthermore, bioactivity in alcohols rapidly increased when the preparations were heated to the boiling point.
Table 3. Bioactivity measurement results of F. esculentum
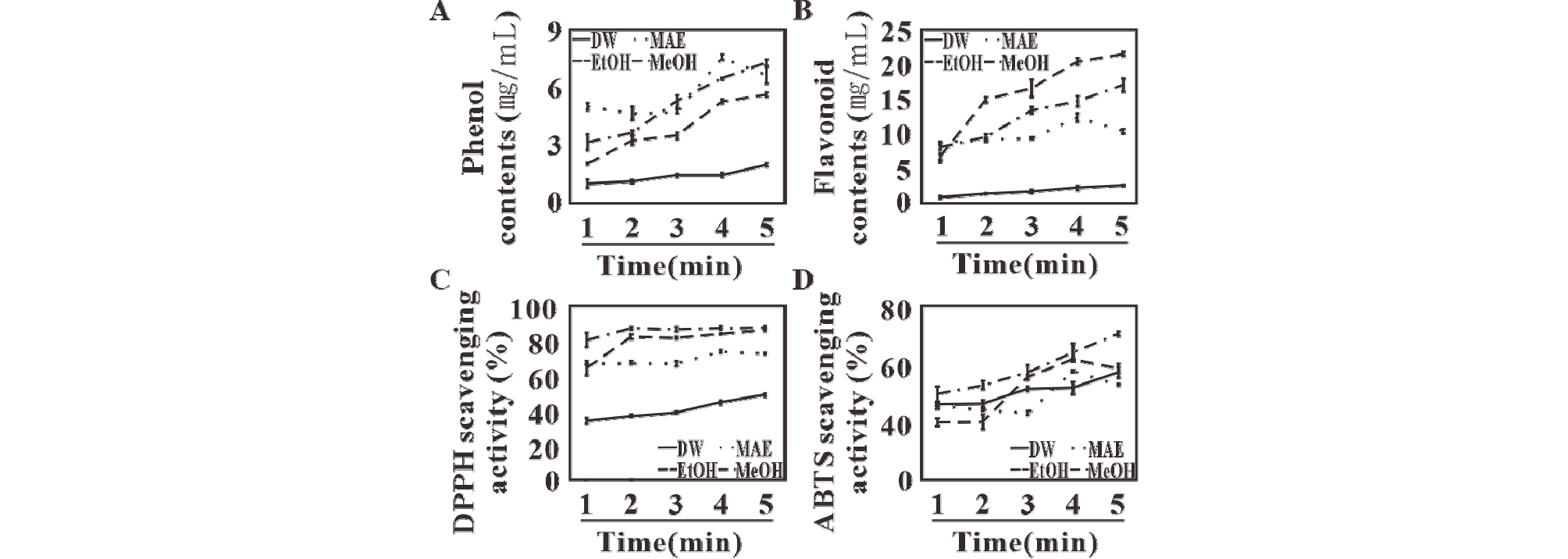
The highest amount of phenol and flavonoids in MAE after four minutes extraction was over four times greater, compared to DWE after five minutes extraction at 90℃ (Fig. 2). In the case of flavonoids, the yield of MAE (11.9 ㎎/mL) was somewhat lower than that of alcohol extraction (E: 20.7 ㎎/mL, M: 16.4 ㎎/mL ), whereas for phenol, the yield of MAE (7.6 ㎎/mL) was higher than that by alcohol extraction (M: 7.3 ㎎/mL, E: 5.7 ㎎/mL). In addition, results for DPPH and ABTS were consistent with those for phenolic contents, i.e., the scavenging ability of DWE (48.4%, 55.6%) was much lower than that of methanol extraction or ethanol extraction (86.7%, 75.6%, and 85.7%, 62.3%, respectively). Nonetheless, MAE (73.0%, 56.2%) showed higher scavenging activity than DWE.
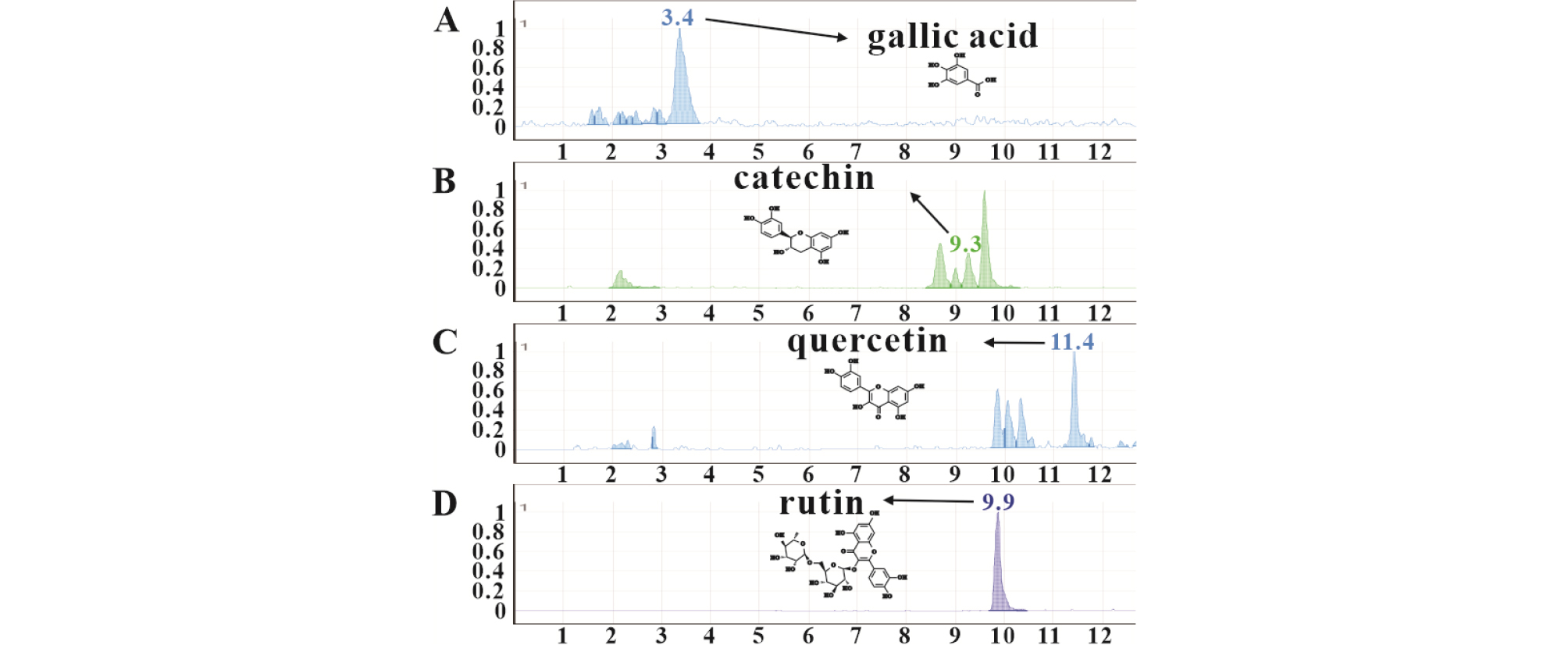
Measurement of the compounds of interest by LC-MS was performed using the conditions shown as most effective in the biological activity experiments for each solvent tested. Thus, in the case of DWE extraction, bioactivity analysis was performed for five minutes at 90℃, and for five minutes at 70℃ for extraction with ethanol and methanol, respectively, while four minutes at 70℃ were used for MAE. The catechins quercetin and rutin are representative water-insoluble phenols, while gallic acid is more soluble than these phenols. The retention times of gallic acid, catechin, rutin and quercetin were 3.4, 9.3, 9.9, and 11.4, minutes, respectively (Fig. 3). Except for gallic acid, measured extracted phenols decreased in the following order: methanol > ethanol > MAE > DWE.
Although extracted gallic acid was lower than other phenols, MAE (206.9 ppb) was more efficient for the extraction of this compound than DWE (204 ppb) or alcohols (103 ppb with ethanol and 97 ppb with methanol).
In the case of water-insoluble phenols (catechins, quercetin, and rutin), amounts extracted with alcohols (2308.3, 688.1, and 9400.4 ppb with ethanol, respectively; 2776.2, 1741.1, and 11591.3 ppb with methanol, respectively) were the highest, whereas DWE allowed minimal extraction of these phenols (4.4, 3.9, and 60.3 ppb, respectively). However, MAE of water insoluble phenols (1204, 110.8, and 2946 ppb, respectively) proved much more efficient than DWE (Table 4).
Table 4. LC-MS analysis of extracts according to solvents of F. esculentum (catechin, gallic acid, quercetin, rutin)
Discussion
Kang’s reported that total contents of phenol and flavonoid of F.esculentum were 13.22 ± 3.69 ㎎/g and 38.53 ± 5.20 ㎎/g, respectively (Kang, 2014). In this study, the contents of phenol and flavonoid were 0.99 ± 0.07~2.02 ± 0.08 ㎎/mL (9.9 ± 0.7~20.2 ± 0.8 ㎎/g) and 0.38± 0.06~2.55 ± 0.09 ㎎/mL (3.8± 0.6~25.5 ± 0.9 ㎎/g), respectively. However, MAE showed 6.07 ± 0.17~7.63 ± 0.14 ㎎/mL (60.7 ± 1.7~76.3 ± 1.4 ㎎/g) and 8.36 ± 0.18~11.89 ± 0.61 ㎎/mL (83.6 ± 1.8~118.9 ± 6.1 ㎎/g), respectively. According to previous study, the free radical scavenging activity for F. esculentum in DWE was 22.18% (Phouthaxay et al., 2015). In this study, the free radical scavenging activity with DWE has shown 20.8 ± 3.0%, whereas for MAE, the free radical scavenging activity has shown 73.0 ± 0.9%. Analysis of useful substances (phenol and flavonoid) and bioactivity(DPPH and ABTS) had shown a similar trend to the previous study in DWE. However, MAE significantly increased rather than DWE. Extraction of MAE was more efficient than that by using other solvents. MAE yielded not only soluble substances, such as soluble phenolic compounds, peptides, proteins, and carbohydrates, (Choi et al., 2006; Liu et al., 2006; Rafiee et al., 2011) but also low-solubility substances, such as insoluble phenolic compounds(rutin, quercetin, etc.) and carbohydrate polymers. Microwaves minimize nutrient loss (Ramesh et al., 2002) because highest yield is conditioned to shortest extraction time possible. Microwaves are efficiently absorbed by polar molecules, resulting in ion conduction and dipole rotation events that can easily rupture cell walls under the influence of internal overheating, vaporization, and decompression, whereby contact between solvents and materials is greatly enhanced. In addition, heat is generated from water and polar compounds present in plant tissues, an effect that contributes to accelerate extraction and improve yield (Chemat and Cravotto, 2012; Ferhat et al., 2007; Vinatoru et al., 2017). Microwaves destroy cell walls and allow external moisture to enter the cell, which in turn facilitates hydration of insoluble phenols. These effects ultimately increase the extraction of useful substances. As a result, MAE without a pretreatment step, such as solvent removal, is considered the most efficient and safer procedure; further, MAE works faster than organic solvents, thereby enabling effective extraction of useful materials from plant tissues. Water extraction using microwaves is effective when it is difficult to extract useful materials using DWE.