Introduction
Materials and Methods
Preparation of plant material
Preculture, loading and dehydration procedure for cryopreservation
Preparation of rootstocks
Micrografting
Optimization of post-culture medium for the recovery of cryopreserved citrus shoot tips
Statistical analysis
Results and Discussion
Effects of preculture conditions on the regrowth rate of cryopreserved Citrus cultivars
Effects of dehydration conditions on regrowth of cryopreserved citrus cultivars
Effects of post-culture media on regrowth of cryopreserved shoot tips of C. limon after micrografting
Introduction
Citrus is one of the most economically important fruit crops and is widely grown in the tropical, subtropical, and even temperate zones of the world (Ding et al., 2008). Historically, Citrus species have been used as fresh fruits, cooking ingredients, medicines, and ornamentals in many countries, including Korea. Citrus germplasm is generally conserved in clonal orchards and valuable genotypes are kept in greenhouses for protection from losses due to pests, diseases, and climatic hazards (Pérez, 2000). The conservation of germplasm in clonal seed banks is difficult to apply, as many of the species have recalcitrant seeds that are sensitive to desiccation (Hay and Probert, 2013; Yi et al., 2015, Yi et al., 2017). Hence, conservation in liquid nitrogen (LN) at -196℃ should be considered as an alternative method for the long-term conservation of Citrus germplasm (De Carlo and Lambardi, 2005).
In vitro micrografting is a relatively new technique in the propagation of plants, which involves the placement of an excised meristem or shoot-tip explant onto a decapitated rootstock that was grown aseptically from seed or micropropagated cultures (Hussain et al., 2014). The technique has great potential for the improvement and large-scale multiplication of fruit plants. It has been used in the production of viral or bacterial disease-free fruit crops, particularly those that are resistant to soil-borne pathogens (Parkinson et al., 1990). For example, Abbas et al. (2008) succeeded in producing Citrus tristeza virus (CTV)-free plants of Kinnow mandarin (Citrus reticulata) and sweet orange (C. sinensis) cultivars using this technique with meristem or shoot tip culture. Natural disasters, however, have the potential to destroy the protective structures that preserve such collections (Volk et al., 2012). Cryopreservation has been found to be a cost-effective and safe method to conserve fruit collections in various genebank centers (Towill et al., 2004). Citrus cryopreservation methods have been developed for a number of different explant types, including shoot tips (Wang et al., 2002a; Wang et al., 2002b; Fifaei et al., 2007; Ding et al., 2008). Numerous studies have investigated Citrus cryopreservation using various explants by different cryopreservation methods (Kaya et al., 2016), however, the cryopreservation of shoot tips was found to be the most reliable cost and space effective method for long-term conservation of clonally propagated germplasm (Engelmann, 2000).
At present, various cryopreservation techniques, such as the droplet-vitrification technique, are available for the long-term conservation of plant genetic resources. Droplet-vitrification is a combination of the vitrification procedure and cooling in droplets of plant vitrification solution (PVS) on aluminum strips for certain plants such as cassava (Gonzalez-Arnao et al., 2008). Various researchers have mainly employed droplet-vitrification for freezing shoot tips obtained from in vitro-grown plantlets (Li et al., 2015; Wang et al., 2017). In this process samples are treated with loading and vitrification solutions and then placed in minute droplets of vitrification solution on aluminum foil strips which are then directly immersed in LN (Sakai and Engelmann, 2007). In recent years, the number of species that have been cryopreserved using the combined droplet-vitrification protocol with rapid cooling and rewarming has significantly increased (Sakai and Engelmann, 2007; Kim et al., 2009a; 2009b; Chen et al., 2011), and include C. sinensis (Sudarmonowati, 2000) and C. madurensis (Cho et al., 2001). The main objective of the present study was to establish a successful cryopreservation and storage technique for shoot tips of two cultivars, ‘Frost Eureka limon’ and ‘Cook Eureka limon’ of C. limon. Micrografting by droplet-vitrification resulted in high regrowth rates after post-thawing.
Materials and Methods
Preparation of plant material
We used C. limon (L.) Burm. f. cultivars ‘Frost Eureka limon’ (IT 234064) and ‘Cook Eureka limon’ (IT 233920), which are widely grown in Korea. The shoot tips were excised from in vitro-grown seedlings. The mature Citrus fruits were collected from the Citrus germplasm field genebank of Jejudo Agricultural Research & Extension Services. These fruits were stored at 4℃ until use. The seeds were thoroughly washed (3-4 times) under running tap water to reduce surface contaminants and then surface sterilized by submerging in 70% (v/v) ethanol for 5 min, followed by 4% sodium hypochlorite (NaOCl) solution for 10 min, and finally rinsed 4-5 times with sterile distilled water (SDW) under laminar airflow. The seeds were inoculated on the seed germination medium (MS salts + 30 g sucrose/L, phytagel 2.6 g/L). The pH of the medium was adjusted to 5.8 prior to autoclaving at 121℃ for 15 min. Seeds were germinated in darkness for 4 weeks and then incubated in a culture room at 25 ± 1℃ under a photoperiod of 16:8 h, light:dark, with an illumination of 45 μmol m-2s-1 from Phillips cool white 18 W fluorescent lamps. After 4-6 weeks of culture, the nodal sections (1.0 ㎝ in length) or shoot tips were inoculated on the shoot multiplication medium composed of Murashige and Skoog (MS) supplemented with 0.2-1.0 ㎎/L 6-Benzyladenine (BA) and 0.1 ㎎/L Kinetin (KN), and incubated in a culture room for 8 weeks under the same previously mentioned conditions. Thus, shoot tips (2.0-2.5 ㎜) were excised from the axillary buds of in vitro-grown plantlets under a stereo microscope (Nikon, SMZ18, Japan) for cryopreservation experiments.
Preculture, loading and dehydration procedure for cryopreservation
Shoot tips were precultured on the MS medium that contained sucrose at 0.3 M and 0.5 M concentrations and incubated for different durations (hours). The various preculture media and time durations were as follows: MS + 0.3 M sucrose for 24 h (Medium 1), MS + 0.3 M sucrose for 24 h and then treated with MS + 0.5 M sucrose for 16 h (Medium 2), MS + 0.3 M Sucrose for 48 h (Medium 3), MS + 0.3 M sucrose for 48 h and then treated with MS + 0.5 M sucrose for 16 h (Medium 4), MS + 0.3 M sucrose for 72 h (Medium 5), MS + 0.3 M Sucrose for 72 h and then treated with MS + 0.5 M sucrose for 16 h (Medium 6). The shoot tips were then treated with a loading solution (17.5% glycerol + 17.5% sucrose in MS) and dehydrated with plant vitrification solution 2 (PVS2: 30% glycerol + 15% DMSO + 15% ethylene glycol + 13.7% sucrose in MS) at 0℃ (Sakai et al., 1990) or plant vitrification solution 3 (PVS3: 50% glycerol + 50% sucrose in MS) for 30, 60, 90, and 120 min at 25℃, prior to direct immersion in LN for 1 h (Kim et al., 2009b). For the droplet-vitrification treatment, five shoot tips (2.0-2.5 ㎜) were transferred to a droplet (3.5 ㎕) of vitrification solution on thin strips of sterile aluminum foil (4.0 × 0.5 ㎝) (Fig. 1c). The aluminum foil strips were then carefully immersed into LN using fine forceps. After immersion, the strips were quickly transferred to 2 ㎖ cryotubes, and immediately plunged into LN.
Preparation of rootstocks
Poncirus trifoliate (L.) Raf. was used as a rootstock. The seeds were extracted from fully matured fruits, washed in tap water, peeled, washed twice with Tween-20 for 3 min each time, washed with distilled water, immersed in 70% ethyl alcohol for 2 min and rinsed with distilled water, followed by surface-disinfecting with 2% sodium hypochlorite (NaOCl) for 10 min. the seeds then were thoroughly rinsed with SDW under laminar airflow and cultured in test tubes that contained 15 ㎖ of MS solid medium (½ MS, 30 g/L sucrose and 2.6 g/L phytagel). The pH of the medium was adjusted to 5.8 prior to autoclaving at 121℃ for 15 min. Seeds were germinated in darkness for 4-6 weeks until use as rootstocks for micrografting.
Micrografting
Micrografting procedure was followed by the method developed by Volk et al. (2012). In brief, the etiolated P. trifoliata seedlings with a height of at least 3 ㎝ were removed from the culture and sliced 1 ㎝ above the cotyledonary node. A 2 mm notch incision was made to bisect the cut surface of the epicotyls and a perpendicular cut was then made to the edge of the seedling (Fig. 1a). After overnight post-culturing on solidified citrus medium, the recovered shoot tips were basally trimmed to create a fresh-cut surface (Fig. 1b) and then placed on the seedling rootstocks (Fig. 1d). Micrografted seedlings were placed on micrografting recovery medium (MS + 30 g/L sucrose + 0.05% activated charcoal + 2.6 g/L phytagel at pH 5.7) in test tubes (20 ㎖ per tube) and cultured at 25℃ under a 16:8 h, light:dark photoperiod provided by fluorescent light (45 μ mol/m/s). Grafted plants were examined at every week. Ten shoot tips of each cultivar were excised and processed for each treatment and each experiment was repeated at least twice. After 6-8 weeks, the micrografted shoots were transferred to fresh media that contained ½ MS with the same components for the formation of roots. Thus fully in vitro-grown plantlets were transplanted into pots that contained organic manure (Seoul Bio Company, South Korea) and that were maintained under greenhouse conditions for their acclimatization.

Fig. 1.
Regrowth from cryopreserved shoot tips of Citrus limon by droplet-vitrification after micrografting. (a) Etiolated ‘trifoliate orange’ seedling rootstocks were prepared by making notched incisions 1.0 ㎝ above the cotyledonary node under a stereo microscope. (b) A typical dissected shoot tip in an enlarged form. (c) Citrus shoot tips contained in droplets of vitrification solution on aluminum foil strips (40 × 5 ㎜). (d) Trimmed shoot tips after post-thaw culture were placed on the notch of the seedling rootstock. (e) Survival of a micrografted shoot tip on the rootstock seedlings of ‘trifoliate orange’. (f) Enlarged form of micrografted shoot tip on the rootstock after 15 days. (g) Recovery of micrografted shoot tips on etiolated ‘trifoliate orange’ seedling rootstocks for 8 weeks. (h) Comparison of non-treated control (con), no liquid nitrogen (LN) exposure (−LN) and LN exposure (+LN) for 6 weeks after micrografting. Ten shoot tips of each cultivar were used for the experiment and each experiment was repeated twice. a, b, d, f: scale bar = 0.5 ㎝; c, e, g, h: scale bar = 1.0 ㎝.
Optimization of post-culture medium for the recovery of cryopreserved citrus shoot tips
Shoot tips of the ‘Frost Eureka limon’ and ‘Cook Eureka limon’ cultivars were precultured using the optimal sucrose concentration. The precultured shoot tips were then loaded and dehydrated under optimal conditions. The shoot tips were immersed in LN (-196℃) for at least 1 h prior to recovery. For recovery, aluminum foil strips were taken out of the cryovials and immediately plunged into a pre-heated (40℃) unloading solution that contained 1.2 M sucrose for 90 s at 25℃ and post-thaw cultured in 20 ㎖ MS medium or woody plant medium (WPM) in Petri dishes (90 mm in diameter) that was supplemented with 1.0 ㎎/L BA and 0.1 ㎎/L KN or without plant growth regulators (PGRs). Four different post-culture media (PCM) used in the post-thaw culture of cryopreserved (+LN) shoot tips of citrus were as follows: MS medium containing NH4NO3 (post-culture medium-1; PCM-1), MS medium without NH4NO3 (post-culture medium-2; PCM-2), woody plant medium (WPM) containing ¼ NH4NO3 (post-culture medium-3; PCM-3), and WPM containing ½ NH4NO3 (post-culture medium-4; PCM-4) without any plant growth regulators. The pH of the media was adjusted to 5.8 before adding 0.05% activated charcoal, and then autoclaved for 15 min at 121℃. The cultures were placed at 24 ± 2℃ in the dark for 1 day and then transferred to light conditions for recovery. The media used in this study were supplied by Duchefa Biochemie, The Netherlands. The regrowth rates (%) of the shoot tips were recorded for 4-6 weeks of the post-thaw culture.
Statistical analysis
Regrowth of the shoot tips was evaluated 4-6 weeks after LN treatment and expressed as the percentage of the shoot tips exhibiting shoot growth. Explants precultured in sucrose and dehydrated with PVS2 but not frozen in LN served as the treated controls (−LN). Two independent experiments were performed with 10 replications per treatment. Significant differences in the treatment means (mean ± SE) for data from each sample were determined using a least significant difference (LSD) at P = 0.05.
Results and Discussion
Effects of preculture conditions on the regrowth rate of cryopreserved Citrus cultivars
The sucrose concentration and duration in preculture medium significantly affected the regrowth rate of the treated control (−LN) and cryopreserved (+LN) shoot tips of the two cultivars (Fig. 2). The regrowth rate was higher in the shoot tips from the treated controls (−LN) from both cultivars. However, the regrowth rate of the cultivar ‘Frost Eureca limon’ (Fig. 2a) was higher than the cultivar ‘Cool Eureca limon’ (Fig. 2b). The regrowth rate decreased significantly when the shoot tips were treated with the MS medium containing sucrose at high concentration (0.5 M) for a longer duration. For cryopreserved (+LN) shoot tips, the highest regrowth rate was obtained from preculturing the shoot tips on medium 4 (Fig. 2). The regrowth rates were 53.5% and 50.3% for cryopreserved shoot tips of ‘Frost Eureka limon’ (Fig. 2a) and ‘Cook Eureka limon’ (Fig. 2b), respectively. Almost all treated control (−LN) shoot tips were regenerated when precultured for 24-72 h in 0.3-0.5 M sucrose concentrations in MS medium. For cryopreserved (+LN) shoot tips, regrowth rate was decreased with longer exposure (48-72 h) to the preculture treatment.
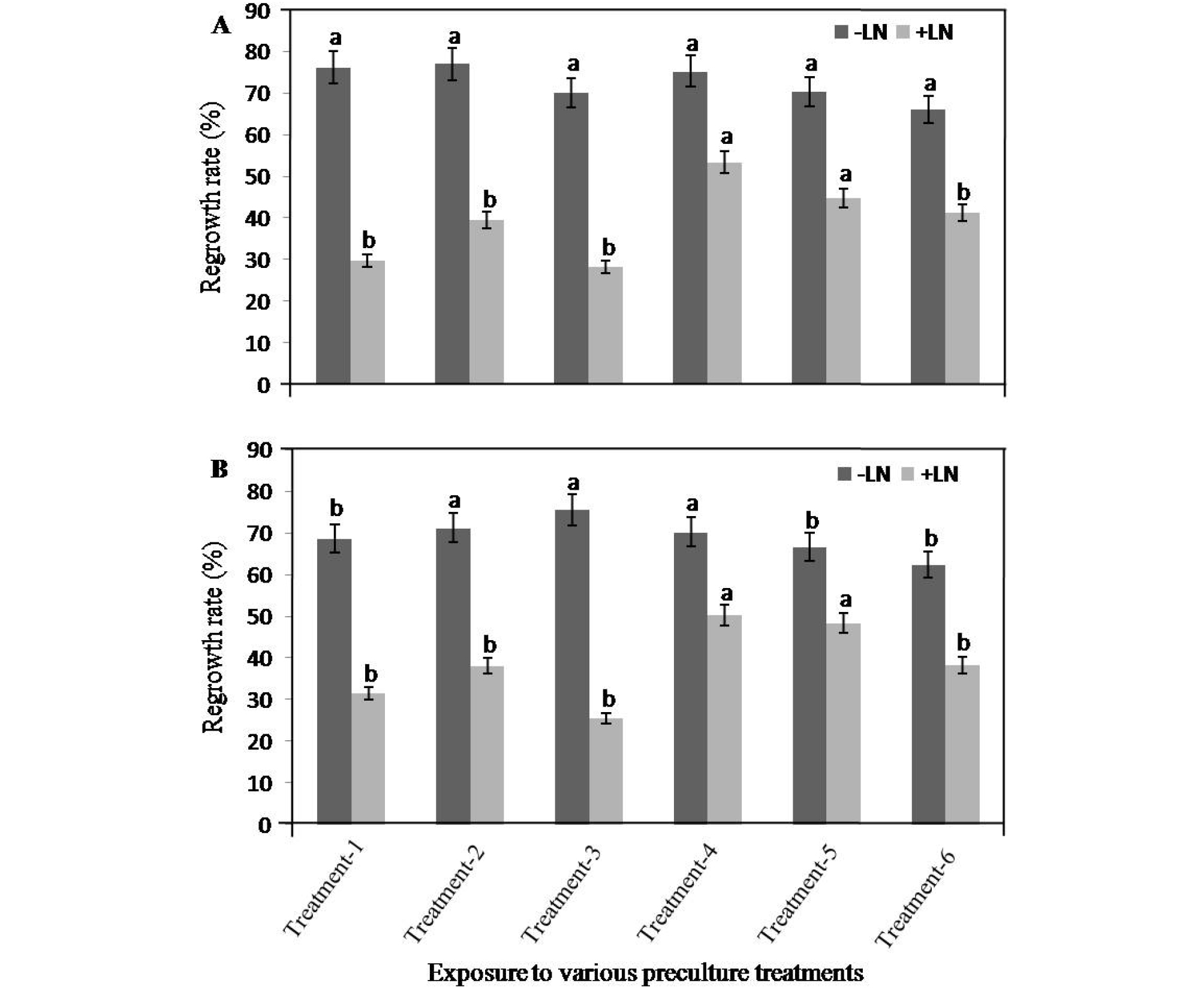
Fig. 2.
Effects of various preculture treatments on the regeneration rates (%) of treated control (−LN) and cryopreserved (+LN) shoot tips of two cultivars: ‘Frost Eureca limon’ (a) and ‘Cook Eureca limon’ (b) of Citurs limon. Shoot tips were pre-cultured on Murashige and Skoog (MS) medium that contained sucrose at 0.3 M and 0.5 M concentrations and incubated for different durations. The various pre-culture media and time durations were as follows: MS + 0.3 M Sucrose for 24 h (Medium 1); MS + 0.3 M Sucrose for 24 h and then treated in MS + 0.5 M sucrose for 16 h (Medium 2); MS + 0.3 M Sucrose for 48 h (Medium 3); MS + 0.3 M sucrose for 48 h and then treated in MS + 0.5 M sucrose for 16 h (Medium 4); MS + 0.3 M Sucrose for 72 h (Medium 5); MS + 0.3 M Sucrose for 72 h and then treated in MS + 0.5 M sucrose for 16 h (Medium 6); followed by loaded and dehydrated with PVS2 at 0℃ or PVS3 at 25℃, prior to direct immersion in LN for 1 h. The results are presented as means ± SE. The same lowercase letters indicate that the bar values do not differ significantly between each other according to LSD (P = 0.05).
In general, treating the samples with preculture solution increased the tolerance of the shoot tips to dehydration during the subsequent freezing in LN. Sucrose concentrations of 0.5-1.0 M have been shown to be suitable for ensuring high survival rates of cryopreserved shoot tips of Malus (Paul et al., 2000) and Poncirus trifoliata×Citrus sinensis (Wang et al., 2002a, b). Sometimes, preculturing causes deleterious effects on shoot tips of some plant species, such as Vitis (Plessis et al., 1991) and P. trifoliata (Gonzalez-Arnao et al., 1998). Our results indicated that Citrus shoot tips of both cultivars were sensitive to a sucrose concentration >0.3 M in MS medium during preculturing, however, all shoot tips tolerated sucrose concentrations as high as 0.5 M for an exposure of 16 h after 48 h exposure of shoot tips in 0.3 M (medium 4). Preculture of explants in media containing sucrose is a valuable method for improving the cryoprotection of shoot tips when stored in LN (Niino et al., 1992). The use of 0.5 M sucrose in the preculture medium was effective for the regrowth of sour orange (Al-ababneh et al., 2002) shoot tips after cryopreservation. On the other hand, Tanaka et al. (2004) stated that higher concentration sucrose solutions cause an osmotic dehydration effect that result in reduced water content in the plant tissue.
Effects of dehydration conditions on regrowth of cryopreserved citrus cultivars
There was no regrowth when the shoot tips were not dehydrated either by PVS2 or PVS3, which indicated that dehydration with PVS is a necessary step for the regrowth of shoot tips of both the ‘Frost Eureca limon’ and ‘Cook Eureca limon’ cultivars in this study. When the shoot tips were treated with PVS2 or PVS3 for different durations of exposure, the regrowth rates were maintained at high levels, which were similar among the 30-120 min durations of exposure in both of the cultivars of the control shoot tips (−LN) (Fig. 3). Regrowth rates of cryopreserved (+LN) shoot tips varied. Regrowth rates decreased when the exposure time exceeded 90 and 120 min in PVS2 and PVS3, respectively. The highest average regrowth rates were 53.5% from cryopreserved (+LN) shoot tips of the ‘Frost Eureca limon’ cultivar when the shoot tips were exposed to PVS2 for 60 min (Fig. 3a), 49.5% when exposed to PVS3 for 90 min in ‘Frost Eureca limon’ cultivar (Fig. 3b). A similar effect was observed in the cultivar ‘Cook Eureca limon’ after exposure to PVS2 or PVS3 for 60 min (Fig. 3c) or 90 min (Fig. 3d), respectively. Optimal time durations of exposure to PVS2 and PVS3 were 60 and 90 min, respectively for regrowth of cryopreserved (+LN) shoot tips from both the cultivars of C. limon. For cryopreserved (+LN) shoot tips, regrowth rates were greatly influenced by the duration of exposure to PVS2 and PVS3.
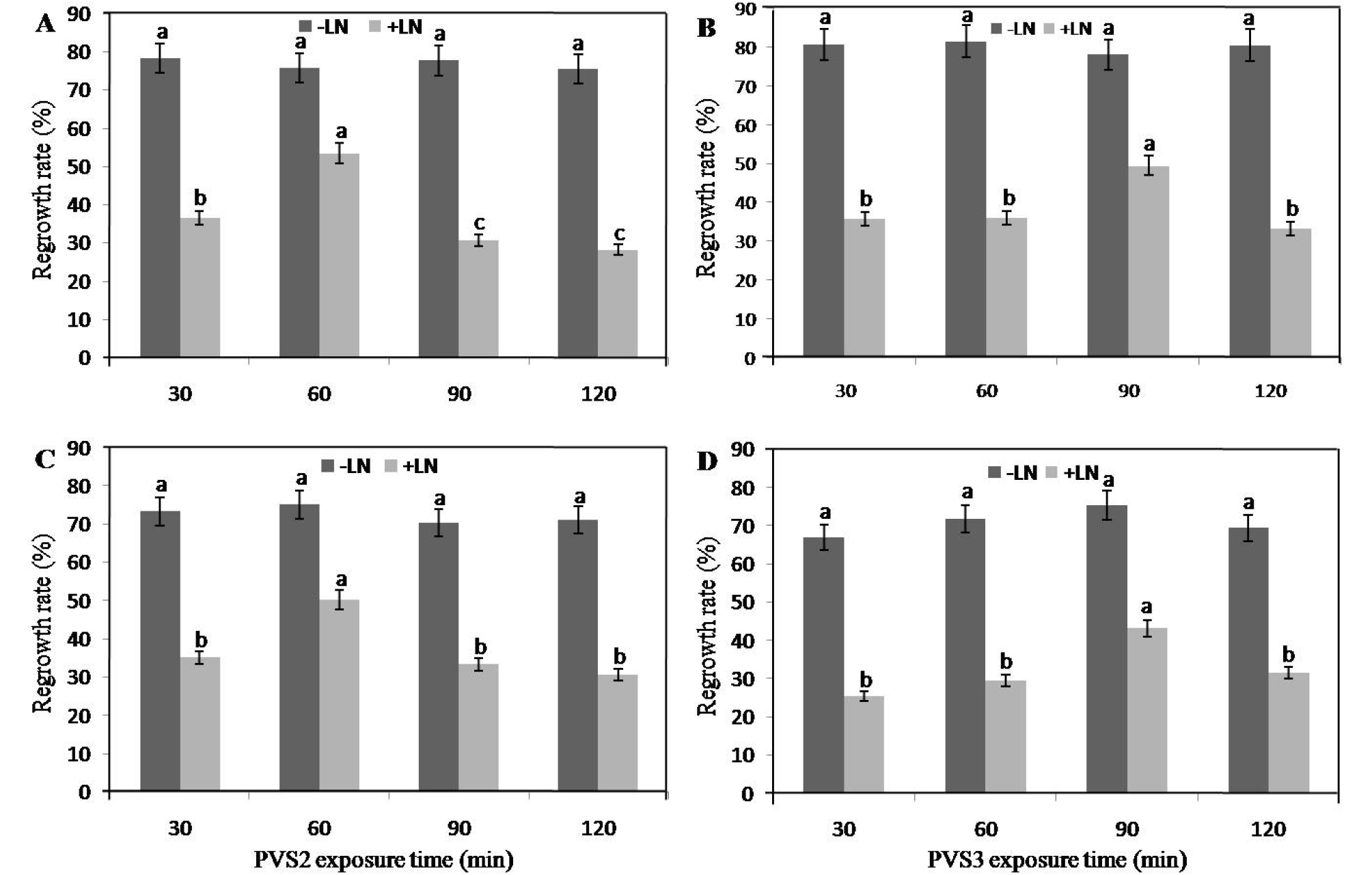
Fig. 3.
Effects of PVS2 and PVS3 exposure on regrowth of the treated control (−LN) and cryopreserved (+LN) Citrus shoot tips in two cultivars cooled to -196℃. Effects of PVS2 and PVS3 duration on regrowth rate (%) of treated control (−LN) and cryopreserved (+LN) shoot tips of citrus cultivars: ‘Frost Eureca limon’ (A & B) and ‘Cook Eureca limon’ (C & D). Pre-cultured shoot tips (MS + 0.3 M Sucrose for 48 h and then treated with MS + 0.5 M sucrose for 16 h) were loaded and dehydrated with PVS2 or PVS3 at 0℃ or 25℃, respectively, prior to direct immersion in LN for 1 h. Regeneration was recorded after 30 days of post-culture. The results are presented as means ± SE. The same lowercase letters indicate that the bar values do not differ significantly between each other according to LSD (P = 0.05). PVS2: 30% glycerol + 15% DMSO + 15% ethylene glycol + 13.7% sucrose in MS; PVS3: 50% glycerol + 50% sucrose in MS.
During a normal cryopreservation procedure by vitrification, precultured shoot tips are dehydrated by exposure to a vitrification solution such as PVS2 prior to direct immersion in LN. Exposure to PVS2 without osmoprotection (loading) is harmful because of osmotic stress or chemical toxicity (Matsumoto et al., 1994). Therefore, loading treatments with glycerol solutions that contain various amounts of sucrose are used, and during this period, plasmolysis occurs which may lead to mechanical stress during dehydration, thus helping shoot tips survive being frozen in LN (Wang et al., 2002b; Hirai and Sakai, 2003). In our study, the droplet-vitrification method was used, in which shoot tips were treated with preculture medium prior to dehydration with PVS2 or PVS3 for various durations. The best regrowth rates of cryopreserved Citrus shoot tips were obtained after exposure for 60 min and 90 min to vitrification solutions, PVS2 and PVS3, respectively for both cultivars. Over-exposure of tissues to PVS2 can cause chemical toxicity and excessive osmotic stress (Wang et al., 2014b). Therefore, optimizing the time of exposure to PVS2 and temperature is necessary for producing a high level of recovery of cryopreserved explants (Sakai et al., 2008). The optimal exposure time with PVS2 varies among plant species and depends on the temperature during exposure (Niino et al., 1997). For a long time, PVS2 has successfully been used to cryopreserve most of the plant species (Sakai and Engelmann, 2007); including citrus shoot tips (Fifaei et al., 2007; Ding et al., 2008).
Previously, Wang et al. (2002b) reported Citrus vitrification methods that used PVS2 as the cryoprotectant and which focused on rootstocks of sour orange (C. aurantium) and ‘Troyer’ citrange (Poncirus trifoliata x C. sinensis). Similarly, this method was used for various Citrus cultivars such as C. sinensis, C. limon, C. reticulata, and C. grandis (Ding et al., 2008). Between the PVS2 and PVS3 solutions for droplet-vitrification, exposure to PVS2 for 60 min was found to most effectively increase regrowth rates after cryopreservation. A previous study by Marković et al. (2013) showed that grapevine shoot tips were highly sensitive to PVS3 exposure which might be due to the presence of an increased concentration of sucrose and glycerol in PVS3.
Effects of post-culture media on regrowth of cryopreserved shoot tips of C. limon after micrografting
In this study, we investigated whether the ammonium ion concentration in PCM affected the viability of cryopreserved Citrus shoot tips. We assessed the viability of cooled samples, following culturing on standard MS, ammonium-free MS, ¼ ammonium WPM, and ½ ammonium WPM medium overnight before micrografting. The recovered micrografted shoot tips that had elongated on recovery medium in about weeks (Fig. 1e, f). The micrografted shoot tips were later successfully grown onto WPM supplemented with activated charcoal (Fig. 1g). There were no significant differences in the regrowth rates among the four media used as controls (−LN) in both cultivars of C. limon (Fig. 4). Activated charcoal in the medium also played a role for successful regrowth rate. For cryopreserved (+LN) shoot tips, the lowest regrowth rates were 53.5% and 50.3% for the cultivars ‘Frost Eureca limon’ and ‘Cook Eureca limon’, respectively by PCM-1, while the highest regrowth rates were 70.3% and 67.3% for the cultivars ‘Frost Eureca limon’ (Fig. 4a) and ‘Cook Eureca limon’ (Fig. 4b), respectively by PCM-3. The optimal PCM for both cultivars (i.e., to increase the regrowth rate) was observed as WPM that contained ¼ NH4NO3 (PCM-3). There were no significant differences in regrowth rates between treatments using PCM-2 and PCM-4. Differences in the comparisons of the non-treated control (con), the treated control (−LN) and LN exposure (+LN) for 6 weeks after micrografting were clearly observed (Fig. 1h).
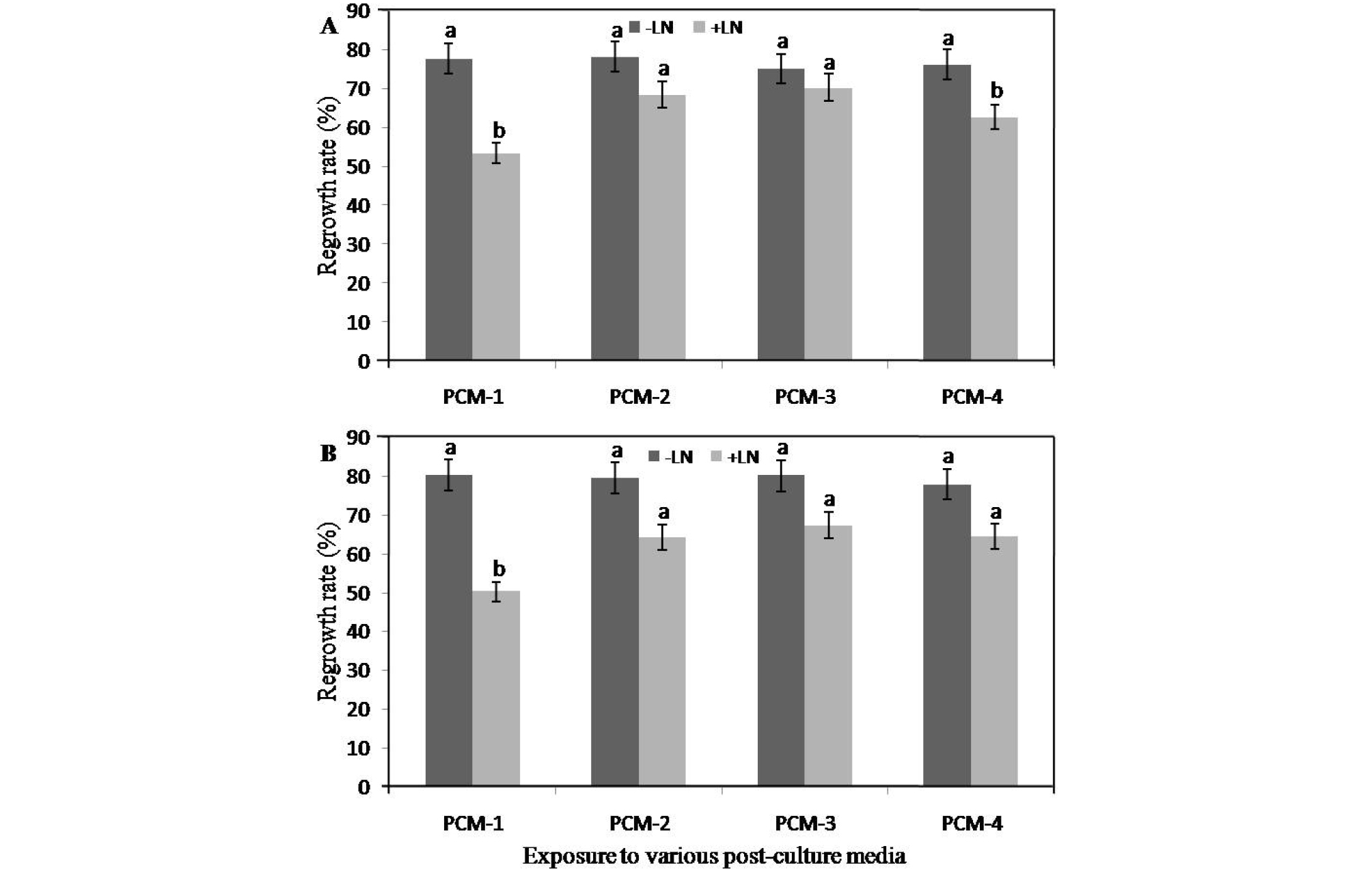
Fig. 4.
Effects of different post-culture media on the recovery of shoot tips of two cultivars ‘Frost Eureca limon’ (A) and ‘Cook Eureca limon’ (B) of Citrus limon after LN exposure. Shoot tips were pre-cultured for 2 d at 24℃ on media with 0.3 M sucrose for 48 h and 0.5 M sucrose for 16 h, followed by a loading solution and dehydration with PVS2 at 0℃ or PVS3 at 25℃, prior to direct immersion in LN for 1 h. Shoot tips were rewarmed at 40℃, unloaded for 20 min in a medium with 0.5 M sucrose, and transferred to Murashige and Skoog (MS) media or woody plant media (WPM) with the following combinations: MS medium containing NH4NO3 (post-culture medium-1; PCM-1), MS medium without NH4NO3 (post-culture medium-2; PCM-2), woody plant medium (WPM) containing ¼ NH4NO3 (post-culture medium-3; PCM-3), WPM containing ½ NH4NO3 (post-culture medium-4; PCM-4), pH of the media was adjusted to 5.8 before adding 0.05% activated charcoal to the medium. The shoot tips un-treated with LN (−LN) served as control. The regeneration rate (%) of the cryopreserved shoot tips was recorded 8 weeks after inoculation. The results are presented as means ± SE. The same lowercase letters indicate the bar values do not differ significantly between each other according to LSD (P = 0.05).
Post-culture recovery medium plays an important role in cryopreserved shoot tips for the regrowth. Very recently, Wang et al. (2017) demonstrated that re-warming in 1.2 M sucrose for 20 min at room temperature, followed by post-thaw culture for a recovery medium that contained 0.3 ㎎/L zeatin increased the regrowth rates of the shoot tips from blueberry plants. Similar results were reported in Citrus and Vitis (Wang et al., 2004), which confirm that the optimal recovery protocol of cryopreserved shoot tips varies among plant species and genotypes. Similarly, during the cryopreservation of Actinidia spp., survival and regrowth increased when cytokinin concentration was increased during the post-thaw recovery stage (Benelli et al., 2013). Studies on rice and lavender cultures demonstrated that the composition of the recovery medium could influence the regrowth of the plants and, in which ammonium played a critical role (Kuriyama et al., 1996). On the other hand, the presence of NH4NO3 in the post-culture media enhanced the regrowth of cryopreserved Holostemma annulare shoot tips (Decruse and Seeni, 2002). Improved regrowth of cryopreserved shoot tips on medium containing ¼ NH4NO3 in the present study was in accordance with the results of these studies. In a conclusion, the cryopreservation protocol for Citrus shoot tips excised from in vitro-grown plantlets of the two cultivars was successfully developed in this study. This cryopreservation protocol using a droplet-vitrification is a promising technique that will promote the establishment of Citrus cryo-banking in Korea.




