Introduction
Materials and Methods
Reagents
Preparation of HF extract
DPPH radical scavenging assay
ABTS cation radical scavenging assay
Total polyphenol content
Cell culture
Cell viability test
Cytokine assay
Histamine assay
Western blot analysis
Statistical analysis
Results
Anti-oxidant Effect of HF extract
Effect of HF on cell viability in HMC-1 cells
Effect of HF on PMACI-induced inflammatory cytokines levels in HMC-1 cells
Effect of HF on PMACI-induced histamine release in HMC-1 cells
Effect of HF on PMACI-induced IκB-a degradation and NF-κB activation in HMC-1 cells
Discussion
Introduction
The skin provides an essential interface between the body and environment. When the skin is exposed to a large amount of ultraviolet rays, high concentrations of reactive oxygen species (ROS) are generated (Blume-Peytavi et al., 2016). Excessive ROS induce lipid damage, DNA oxidation and increase of inflammatory factors, which ultimately causes skin inflammation (Godic et al., 2014). Therefore, maintain a continuous supply of antioxidants in the skin can help prevent skin inflammatory diseases.
Mast cell activation play an important role in skin diseases such as allergic contact dermatitis, psoriasis, and atopic dermatitis (Gentek et al., 2018). In chronic inflammatory response, mast cell generates a variety of cytokines and chemokine, including tumor necrosis factor (TNF)-α, tryptase, interleukin (IL)-8, histamine, and other chemokines, leading to tissue damage (Galli et al., 2005). The increase in these factors may be critical to the development of inflammatory disorder (Trefzer et al., 2003). Therefore, suppression of excess inflammatory cytokines may contribute to the development of a useful therapeutic strategy against inflammatory diseases.
Nuclear factor-kB (NF-kB) is a transcription factor that is vital in regulating the expression of various genes involved in immune and allergic inflammation (Lappas et al., 2002). During inflammation, the IκB kinase complex is phosphorylated and degraded. In turn, the activated NF-κB is translocated into the nucleus, inducing the transcription of inflammatory genes. Increased NF-kB activity associated, which is associated with the secretion of high of IL-6 and TNF-a levels, is involved in skin inflammation (Lee et al., 2020). These results demonstrate that modulating of NF-kB pathway is as an attractive target for treating inflammatory skin diseases.
Recently, published studies have demonstrated the various pharmacological applications of marine plants, which are now used worldwide as functional materials (Jeon et al., 2021). Hijikia fusiforme (HF), a member of brown algae family that exerts various biological effects, including preventing arteriosclerosis and menopausal disorders (Jeong et al., 2015; Kwon et al., 2016). However, a precise understanding of mechanism of action of HF on mast cell-mediated inflammatory response is lacking. This study aimed to elucidate the ameliorative effect of HF on skin inflammation. We investigated the antioxidant and anti-inflammatory effect of HF extract to evaluate its potential as a functional materials.
Materials and Methods
Reagents
Gallic acid, bicinchoninic acid (BCA), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), potassium persulfate, dimethyl sulfoxide (DMSO), Folin-Denis, avidin peroxidase (AP), 2,2-diphenyl-1-picrylhydrazy (DPPH), phorbol 12-myristate 13-acetate (PMA), 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) were obtained from Sigma Chemical Co. (St. Louis, MO, USA). The ELISA assay kits for human IL-8/ IL-6/TNF-α were procured from BD Biosciences (CA, USA). IκB-α and NF-κB specific antibodies (Abs) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Preparation of HF extract
HF was obtained from Wando local market and authenticated by Dr. Noh-Yil Myung (Wonkwang Digital University). The HF (100 g) was decocted in 1 L of distilled water, and filtered, freeze dried, and stored at 4˚C (yield, 7.42%). The sample was dissolved in PBS and filtered through a 0.22 ㎛ syringe filter.
DPPH radical scavenging assay
The DPPH- free radical scavenging ability of HF was determined according to the method described by Naczk and Shahidi (2006). Briefly, 100 µL of HF at different concentrations (0.1 ㎎/mL, 0.5 ㎎/mL and 1 ㎎/mL) were introduced into in a 96-well plate and 100 µL of solution of DPPH in ethanol were added. The mixture was reacted in the dark. Absorbance was evaluated at 520 ㎚ using a Molecular Devices microplate reader.
ABTS cation radical scavenging assay
ABTS cation radical scavenging activity was determined as per the method described by Re et al. (1999) with slight modification. Briefly, 7.6 mM ABTS and 2.4 mM potassium persulfate were mixed in 1:1 ratio by volume and oxidized in the dark for 24 h. An ethanol gradient dilution was performed so the absorbance of the ABTS working solution at a wavelength of 730 ㎚ was 0.7 ± 0.03. Then, 0.5 mL of HF at concentrations (0.1 ㎎/mL, 0.5 ㎎/mL and 1 ㎎/mL) was added to 0.5 mL of the ABTS+ working solution, shaken well, and then kept in the dark for 10 min. Finally, 200 μL of this mixture were dispensed into a plate, and then absorbance at 730 ㎚ was measured.
Total polyphenol content
The total polyphenol content of the extract of the sample was measured using a Folin-Denis reagent. Briefly, 0.5 mL of the HF extract was added to each tube and Folin-Denis reagent (0.5 mL) was subsequently added and allowed to react for 3 min. Subsequently, 3 mL of 10% Na2CO3 was added, mixed and incubated in the dark for 30 minutes, and then the absorbance of the samples was measured. The total polyphenol content was calculated using a typical calibration curve (R2 ≥ 0.99) prepared with garlic acid as the standard.
Cell culture
Cells were maintained in IMDM containing with penicillin (100 IU/ml), streptomycin (100 mg/ml), and 10% FBS at 37℃ in 5% CO2 atmosphere. HMC-1 was activated with of PMA (50 nM) plus the calcium Ionophore A23187 (1 ㎎/mL).
Cell viability test
To investigate the cell viability at various HF concentrations, an MTT colorimetric assay was conducted. Briefly, cells were incubated with HF (0.1, 0.5 and 1 ㎎/mL) for 12 h and MTT (50 L) was subsequently added. After 4 h incubation, formazan was dissolved with DMSO, and the absorbance was measured at 540 ㎚ by a microplate reader (Molecular Devices, CA, USA).
Cytokine assay
The levels of human IL-8, IL-6 and TNF-α was evaluated by modified enzyme-linked immunosorbent assays (ELISA) as previously described (Kee and Hong, 2019). Briefly, micro plates were coated with monoclonal Abs of anti-human IL-8, IL-6 and TNF-α overnight at 4℃. After additional washes, the samples or standard solution of IL-8, IL-6 and TNF-α was added. After washes, the plates were incubated with biotinylated Abs and incubated for 2 h. After washing the plates, AP and substrates were sequentially added; after color development was read at 405 ㎚.
Histamine assay
Histamine concentration derived in mast cell was evaluated with a histamine assay kit (Neogen, Lexington, USA) following the manufacturer’s protocol.
Western blot analysis
Nuclear lysates were prepared using nuclear extraction reagent kit (Pierce Thermo Scientific, IL, USA) as per the manufacturer’s instructions. After protein quantification, the sample was mixed with 2x sample buffer, separated by gel electrophoresis and transferred onto membranes. Membrane was blocked by 5% skimmed milk and subsequently reacted with NF-kB p65 primary Abs. After washing, membrane was reacted with secondary Abs for 1 h. After further washing, protein bands were visualized using an ECL detection system (Thermo Fisher Scientific. NJ, USA).
Statistical analysis
Results are shown as the mean ± SD of three parallel experiments. The statistical analyses were examined using an independent t-tests and one-way ANOVA with a Tukey post hoc test. P < 0.05 was considered significant difference.
Results
Anti-oxidant Effect of HF extract
Natural antioxidants reduce the oxidation of DPPH and ABTS radicals. The DPPH and ABTS radical scavenging assay is widely used method to evaluate the antioxidant activity. DPPH and ABTS radical scavenging activities of HF at different concentration are shown in Fig. 1A and B. We observed that the DPPH radical scavenging ability of HF exhibited nearly 35.8% (0.1 ㎎/mL), 56.2% (0.5 ㎎/mL), and 76.8% (1 ㎎/mL), respectively. In the evaluation of ABTS radical scavenging ability, HF extracts showed efficacies of 30.5% (0.1 ㎎/mL), 42.3% (0.5 ㎎/mL), and 69.2% (1 ㎎/mL), respectively (Fig. 1B).
Phenolic compounds are secondary metabolites, widely distributed in the plant. They have various physiologically active functions such as antioxidant, antibacterial, and anti-inflammatory actions, and are used as functional ingredients. The antioxidant activity of natural products is correlated with polyphenol contents. In this study, Folin-Denis method was modified to measure the total polyphenol content by concentration of HF extracts. As a result, the total polyphenol content of HF (1 ㎎/mL) was 70.2 ㎎ GAE/g (Fig. 1C). Thus, we verified that HF extract has antioxidant activity.

Fig. 1.
Antioxidant effects of HF. Antioxidant effects was evaluated by (a) DPPH free radical scavenging assay, (b) ABTS+ cation radical scavenging assay, (c) Total polyphenol contents. The Antioxidant activity of HF were prepared as described in the Materials and Methods section. Results are shown the mean ± SD of experiments. #, P < 0.05; significantly different from control (vehicle).
Effect of HF on cell viability in HMC-1 cells
To elucidate its anti-inflammatory effect and mechanism of action, cell viability analysis was performed to determine the cytotoxicity of HF extracts. The cells were incubated with or without HP (0.1, 0.5 and 1 ㎎/mL) for 24 h, and the cytotoxic effects of HF was evaluated using a MTT reagent. No cytotoxicity due to HF was observed in our experiments (Fig. 2).
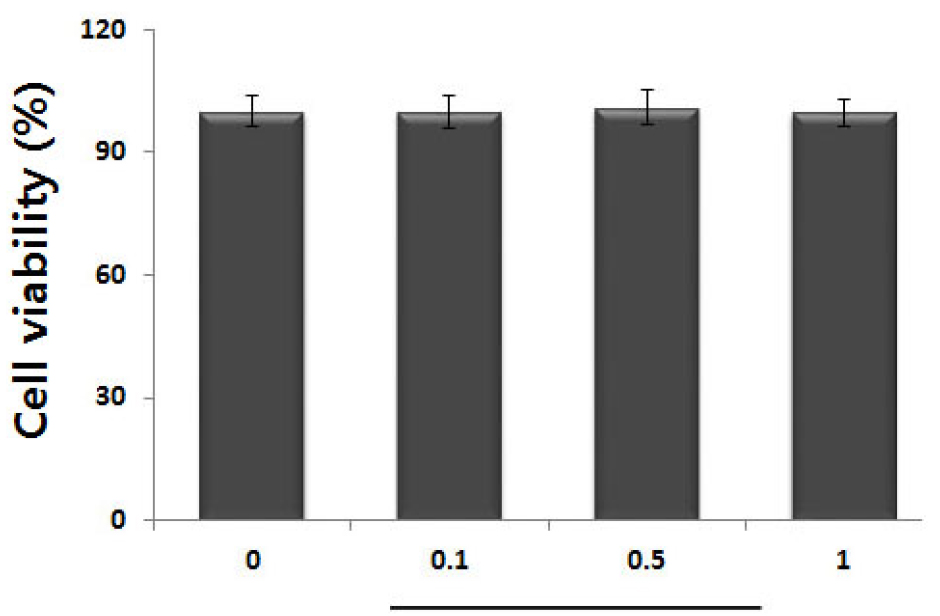
Fig. 2.
Effect of HF on cell viability in activated HMC-1 cells. (A) Cells (3 × 105 cells/well) were treated with various concentration of HF (0.1, 0.5 and 1 ㎎/mL) for 12 h and cell viability was measured using the MTT assay. Results are shown the mean ± SD of experiments. #, P < 0.05; significantly different from control (vehicle).
Effect of HF on PMACI-induced inflammatory cytokines levels in HMC-1 cells
Inflammatory cytokine level attenuation is a therapeutic strategies for inflammatory disease (Fedenko et al., 2011). Therefore, we determined the inhibitory effect of HF on IL-8, IL-6 and TNF-α secretion in PMACI-activated HMC-1 cells. The cells were incubated with or without HF (0.1, 0.5 and 1 ㎎/mL) and activated with PMACI for 7 h. The results showed that PMACI alone induced IL-8, IL-6 and TNF-α levels in PMACI- activated HMC-1cells. However, HF attenuated the PMACI-enhanced the IL-8, IL-6 and TNF-α levels in a concentration-dependent manner (Fig. 3). The maximal inhibitory rate of IL-8, IL-6 and TNF-α secretion by HF (1 ㎎/mL) was approximately 26.2% (P < 0.05), 38.6% (P < 0.05), and 35.2% (P < 0.05), respectively.
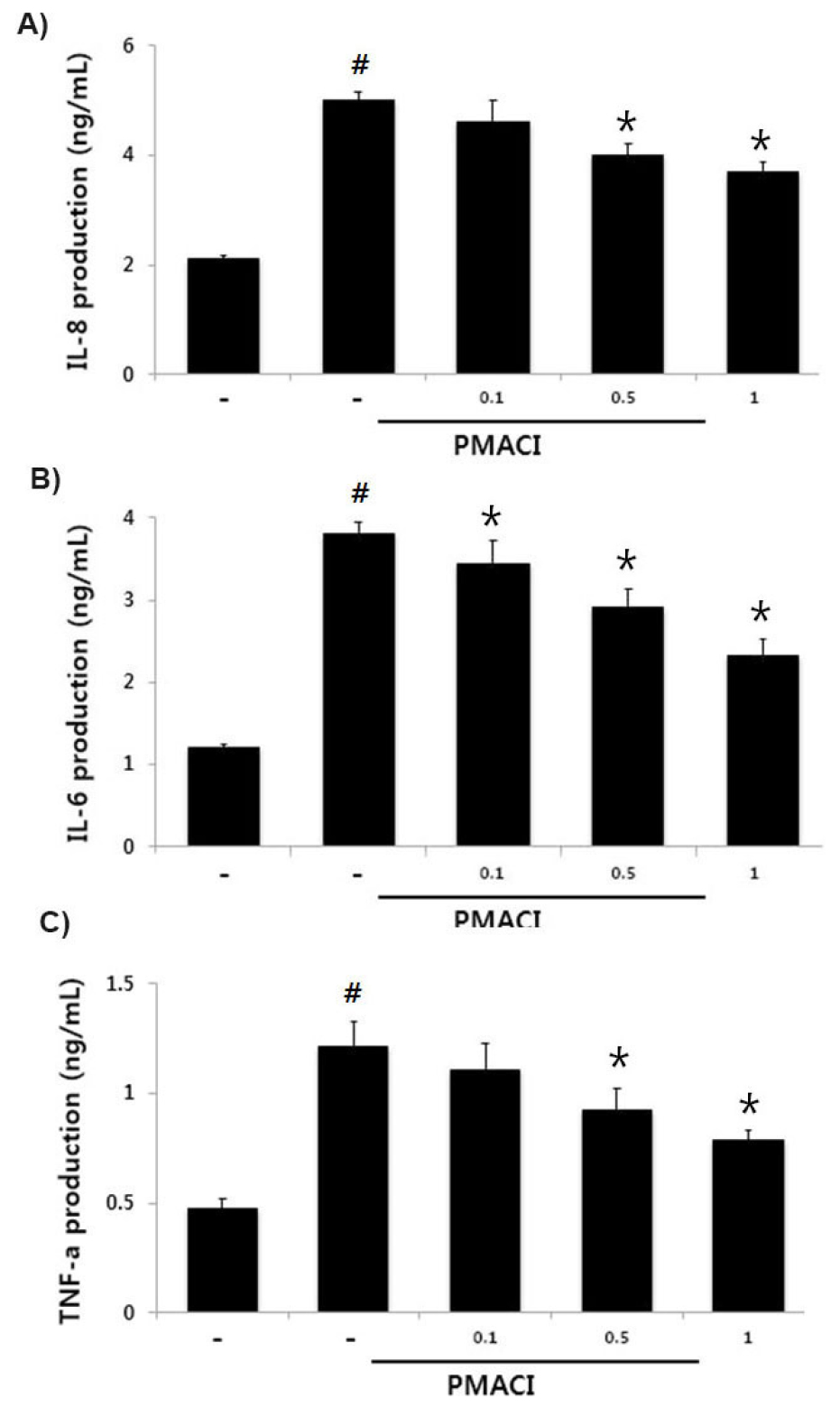
Fig. 3.
Effects of HF on IL-8, IL-6 and TNF-α production in activated HMC-1 cells. Cells were incubated with various concentration of HP (0.1, 0.5 and 1 ㎎/mL) for 1 h before stimulation with PMACI for 7 h. The IL-8, IL-6 and TNF-α concentration were determined by ELISA kits. Results are shown the mean ± SD of experiments. #, P < 0.05; significantly different from control, *, P < 0.05; significantly different from PMACI alone treatment.
Effect of HF on PMACI-induced histamine release in HMC-1 cells
Mast cell-derived histamine initiates allergic inflammation and its appropriate regulation may help treat allergic inflammation (Jemima et al., 2014). Thus, we measured the beneficial effect of HF on PMACI–enhanced the histamine release using histamine assay kit. As a results, HF decreased PMACI-induced histamine levels in a concentration-dependent manner (Fig. 4), suggesting that HF exerts an anti-allergic inflammatory effect through suppressing of histamine secretion. The maximal inhibitory rate of histamine amount by HF (1 ㎎/mL) was approximately 34.4% (P < 0.05).

Fig. 4.
Effects of HF on histamine levels in activated HMC-1 cells. Cells were treated with various concentration of UP (0.1, 0.5 and 1 ㎎/mL) for 1 h before stimulation with PMACI for 2 h. The histamine concentration was measured by histamine assay kit. Results are shown the mean ± SD of experiments. #, P < 0.05; significantly different from control, *, P < 0.05; significantly different from PMACI alone treatment.
Effect of HF on PMACI-induced IκB-a degradation and NF-κB activation in HMC-1 cells
Since NF-kB suppression has been linked to anti-inflammatory response (Birrell et al., 2005), we predicted that NF-kB activation attenuation might be the molecular mechanism of HF. Since inhibitors of NF-kB exert their effects by inhibition the IκB-α degradation in the cytosol, we investigated the IκB-α levels in the cytosol after PMACI treatment using a western blot analysis. The results showed that PMACI treatment effectively induce IκB-α degradation, but HF significantly inhibited PMACI-induced IκB-α degradation in the cytosol and relative of IκB-α levels are shown in Fig. 5A. As NF-kB activation requires nuclear NF-kB translocation, the effects of HF on the nuclear pool of the RelA/p65 protein were assessed in the nucleus. In PMACI - activated cells, the RelA/p65 levels were increased; however, HF attenuated the enhanced RelA/p65 levels and relative of NF-kB levels are shown in Fig. 5B.
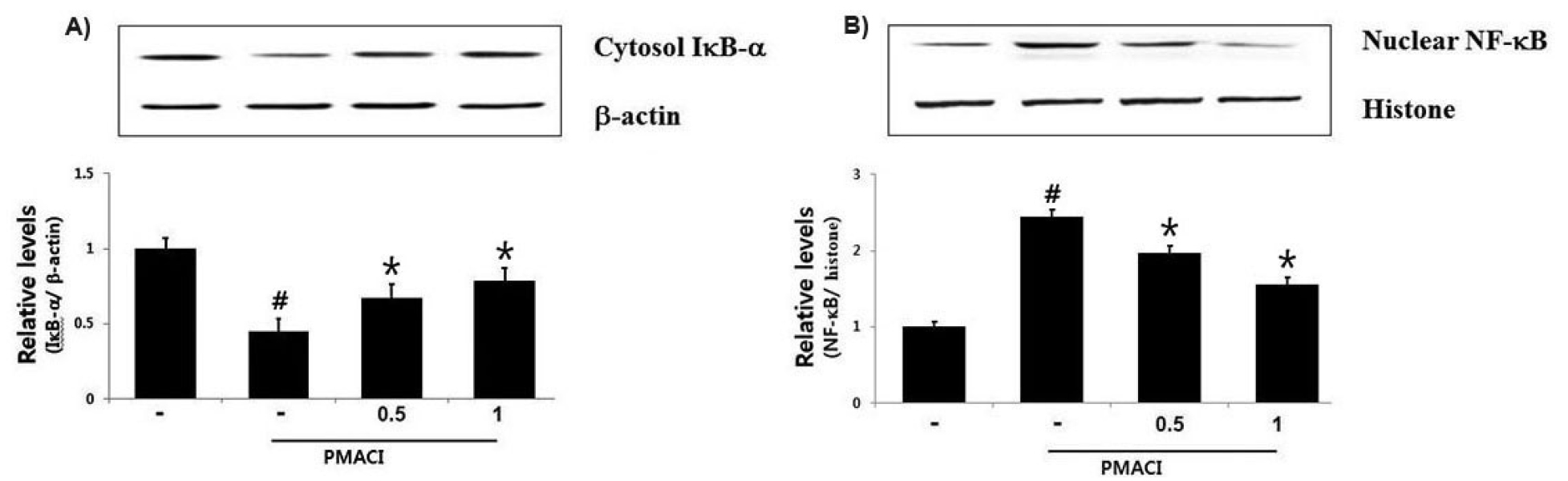
Fig. 5.
Effect of HF on IκB‐α degradation and NF-κB activation in HMC-1 cells. Cells (6 × 106 cells/well) were incubated with various concentration of HF (0.5 and 1 ㎎/mL) for 1 h and then activated with PMACI for 2 h. (A) Cytosolic extracts were prepared and evaluated for IκB‐α expression by Western blot analysis and IκB‐α relative level is presented. (B) Nuclear NF-κB were evaluated by Western blot analysis and NF-κB relative level is presented. Results are shown the mean ± SD of experiments. #, P < 0.05; significantly different from control, *, P < 0.05; significantly different from PMACI alone treatment.
Discussion
Marine plants have been used worldwide to improve human health promotion. While the precise mechanisms of action of marine plants remain to be elucidated, further study on identifying novel therapeutics with immunomodulatory activities are essential. HF, a member of brown algae family, has a major constituents including fucoidan and laminaran, which have biological effect of immune regulation and prevention of cholesterol deposits. In this study, we demonstrated the antioxidant and anti-inflammatory effect of HF extract, thereby validating its potential as a functional material. The study demonstrates that HF has strong free radical scavenging activity. HF attenuated the PMACI-induced IL-8, IL-6, TNF-α and histamine secretion in activated HMC-1cells. Additionally, HF exhibited inhibitory effects on PMACI-stimulated HMC-1 cells by suppressing IκB-a degradation /NF-kB activation.
The skin is always in contact with various environmental factors (Proksch et al., 2008). Prolonged skin exposure to ultraviolet rays leads to the generation of high ROS concentrations, causing the antioxidant defense system to collapse (Aroun et al., 2012). The resulting oxidative stress leads to cell damage, thereby accelerating skin aging and inflammation (Addor, 2017). Antioxidants play an essential role in human body to reduce oxidative processes and the harmful effects of free radicals. Natural antioxidants can be considered as potential therapeutic agents against many diseases. To elucidate the anti-oxidative activity of HF, we test the regulatory effect of HF at different concentration on DPPH- and ABTS- radical scavenging activities. Consequently, HF demonstrated strong scavenging effects on both ABTS and DPPH free radicals. The total polyphenol content of HF (1 ㎎/mL) was 104.17 mg GAE/mg (Fig. 1C). Thus, we suggest that the HF extract has strong antioxidant activity.
Mast cell activation play an important role in skin diseases such as allergic contact dermatitis, psoriasis, and atopic dermatitis (Caughey, 2016). In chronic inflammatory response, mast cell generates a variety of cytokines and chemokine, including tumor necrosis factor (TNF)-α, tryptase, interleukin (IL)-8, histamine, and other chemokines, leading to tissue damage (Li et al., 2019). An increase in the levels of these factors may prove critical for the development of in inflammatory disorder (Yao and Narumiya, 2019). MC-derived IL-8 is a chemotactic factor for eosinophil and neutrophil, which activates inflammatory response (Olivera et al., 2018). TNF-α secreted from mast cells, accumulates white blood cells, resulting in inflammation response (Fedenko et al., 2011). Moreover, histamine plays a central role in the pathogenesis of allergic asthma, allergic rhinitis and atopic dermatitis through the release of leukotrienes, cytokines, and chemokines (Jemima et al., 2014). Therefore, modulating of excess inflammatory genes is a useful therapeutic strategy against inflammation. In this study, we demonstrated that HF attenuated the production of IL-8, IL-6, TNF-α and histamine in activated HMC-1 cells. The inhibitory rates of IL-8, IL-6 and TNF-α by HF (1 ㎎/mL) were approximately 34.05 %, 30.83 %, and 36.07%, respectively. In addition, HF decreased the PMACI-enhanced histamine production in a concentration-dependent manner (Fig. 3). These results suggest that HF exerts an anti-inflammatory effect by suppressing the production of mast-cell-derived inflammatory mediators.
Emerging evidence has shown that increases of inflammatory genes are associated with NF-κB pathway (Sakamoto et al., 2018). During inflammation, the IκB kinase complex phosphorylates and degrades the IκB, allowing NF-κB to translocate to the nucleus and bind to promoters to induce the expression of inflammatory genes (Lappas et al., 2002; Sakamoto et al., 2018). Anti-inflammatory agents suppressed NF‐κB activation by stabilizing IκB‐α. Therefore, NF-kB pathway regulation is as an ideal target for treating inflammation. To explore the anti-inflammatory mechanism of HF, we investigated whether HF could attenuate the IκB-α degradation in the cytosol and nuclear NF-kB translocation. We found that HF inhibited PMACI-induced the IκB-α degradation and NF-kB translocation in activated HMC-1 cells. Hence, our results suggest that the beneficial effects of HF on mast cell-related inflammation may be due to the regulating of the NF-κB pathway in activated- HMC-1 cells.
In conclusion, HF has anti-oxidative and the anti-inflammatory activity, attributable to the scavenging of ABTS and DPPH free radicals and suppression of inflammatory cytokines in HMC-1 cells. Additionally, the mast cell-mediated anti-inflammatory mechanism of HF results from attenuating IκB-α degradation and NF-kB activation. Our novel results provide evidence that HF is a potential therapeutic agent for inflammation.


