Introduction
Materials and Methods
Plant material
Spore germination in vitro
Gametophyte proliferation
Sporophyte formation
Statistical analysis
Results and Discussion
Effect of medium on gametophyte proliferation
Difference in the development of gametophytes according to medium
Effect of soil types on sporophyte production
Introduction
The Asian chain fern [Woodwardia japonica (L. f.) Sm.], native to Korea, is an evergreen perennial that is classified as a large type species among pteridophytes. It is present only in Asia, in countries such as China, Japan, and Vietnam, and a few are found in Jeju and Jeollanam-do in Korea. The rhizome is thick and stands at an angle, with dense brown lanceolate scales. The leaves are radical and the petioles are thick, hard, and 30-50 ㎝ long. The leaf blade is elliptical, ovate, 40-80 ㎝ long, 20-35 ㎝ wide, and split. Split leaves appear in 10-15 pairs, and are linear, lanceolate, feather shaped, split to the center, pointed, and green. The leaf backside is light green, with brown scales on the veins. The sorus is 2-5-㎜ long, close to the central leaf vein, and spore formation occurs from July to September (KBIS, 2019).
Habitats of the Asian chain fern are rich in litter layers in forests in the mountains; thus, the fern can grow in shade. Recently, owing to its ornamental value the collection of Asian chain fern has been indiscriminate. As a result, only young individual plants are fund in their natural habitats (KBIS, 2019); in 2017, it was categorized under endangered wildlife class II and evaluated as a vulnerable species (VU), which is an endangered category in the Korean Red List (BK, 2019). Therefore, a preservation plan for this species is urgently required.
Ferns are increasing in demand due to their high utilization. Some species, such as Microlepia strigose (Cho and Lee, 2017), Cheilanthes argentea, Athyrium sheareri (Jang et al., 2019a; 2019b), Arachniodes aristata (Cho et al., 2017), Polystichum braunii (Kwon et al., 2017), and Leptogramma pozoi (Lee et al., 2019) have a mass production system. However, most of these systems are not well established. In addition, because pteridophytes are unlike spermatophytes, suitable proliferation methods must be determined at each of the two growth stages, namely, the gametophyte and sporophyte. Tissue culture techniques and container propagation methods must be performed in parallel to achieve effective growth.
The purpose of this study was to investigate suitable conditions for gametophyte proliferation and sporophyte production that produce a large number of individuals in order to preserve the species and enhance the utilization of the ornamental value of the Asian chain fern.
Materials and Methods
Plant material
Plants were collected from Seogwipo-si, Jeju-do, South Korea (lat. 33°18′0.59″N, long. 126°34′31.9″E) and transplanted into a plastic-film greenhouse under 70% shading at Chungbuk National University, Cheongju-si, Korea (lat. 36°37′29.0″N, long. 127°27′18.4″E). Sporophylls were harvested on August 18, 2014 and spores were selected using the method of Cho and Lee (2017). Collected spores were sealed in a vial bottle and stored at a low temperature (4 ± 1℃).
Spore germination in vitro
To obtain gametophytes for the experiment, spore germination was performed according to methods described by Cho et al. (2017). The supernatant was removed by centrifugation (HA-12, Hanil Science Industrial, Incheon, Korea) for 3 min in a suspension of 50 ㎎ of selected spores immersed in 15 mL of distilled water for 24 h. This was sterilized for 13 min by adding 1.4% sodium hypochlorite solution, and then this was washed five times with sterile water. The spore solution was prepared by adding 15 mL of sterile water to the sterilized spores.
For spore germination, 30 mL of Knop medium, supplemented with 3.0% (w/v) sucrose and 0.8% (w/v) agar and adjusted to pH 5.8, was dispensed in 8.9 ㎜ Petri dishes. After the mixture solidified, 1 mL of sterile water and 1 mL of prepared spore solution were inoculated together. The spores were germinated at 25 ± 1℃ under a light intensity of 30 ± 1.0 μmol·m-2·s-1 (16/8 h photoperiod). Gametophytes obtained from germinated spores were subcultured in MS medium at 2-week intervals.
Gametophyte proliferation
MS medium, adjusted to concentrations of 1/8, 1/4, 1/2, 1, and 2 × the original concentration, and Knop medium (0.5% sucrose, 0.8% agar, and pH 5.8) were applied. Gametophytes in a uniform state and secured by subculture were chopped into 300 ㎎ pieces using a scalpel and inoculated into prepared media, followed by the addition of sterile water. The inoculated media bottles were placed in a culture room at 25 ± 1.0℃ under a light intensity of 43 ± 2.0 μmol·m-2·s-1 (16/8 h photoperiod). All treatments were performed in four replicates, and after 8 weeks of incubation, the fresh weight, development, and morphogenesis of each gametophyte were observed using an electronic scale and a stereomicroscope.
Sporophyte formation
To establish suitable substrate conditions, methods using blender (Cho and Lee, 2017) were referred to for sporophyte formation. Eight soil mixtures of the same composition used in Cho et al. (2017) study were prepared using horticultural substrates, peat moss, perlite, and decomposed granite (Table 1), and the chemical properties of each soil were the same as in the results of Cho and Lee (2017). The physical properties of each soil are: commercial bed soil (horticultural substrates, Hanareum no. 2; Shinsung Mineral Co., Ltd., Korea), 60-80% water holding capacity; peat moss (SunShine, Sun Gro Horticulture, Canada), 94.2-95.2% porosity; perlite (Newpershine no. 2; GFC. Co., Ltd., Korea), 20% available water, pH 6.0-7.0, over 1000 ㎜·Hr-1 coefficient of permeability; decomposed granite (Samgye Masato, Korea), 2 ㎜ particle size.
Table 1. Soil compositions used for sporophyte production
Square pots (75 × 75 × 75 ㎜) were filled with the prepared soil mixtures and placed in a plastic box (503 × 335 × 195 ㎜). In vitro-cultured gametophytes were sterilized for 1 h with a 1000 × fungicide solution (Hymexazol 30%; Tachigaren, Dongbu Agrotech, Korea) and washed five times with distilled water. Sterilized gametophytes (1 g) were added to 25 mL of distilled water in a plastic beaker and were ground for 10 s using a hand blender (V-8000, Boowon, Korea). Then, ground gametophytes were spread uniformly on top of the soil mixtures and the boxes were covered with glass plate. These plastic boxes were incubated for 10 weeks at 25 ± 1℃ under a light intensity of 43 ± 2.0 μmol·m-2·s-1 (16/8 h photoperiod).
During incubation, subirrigation was performed to a height of 1 cm in each plastic box, and a relative humidity of 72 ± 2% was maintained. After a young gametophyte formation was observed, water was sprayed daily on the surface of the gametophyte to facilitate the formation and development of sporophytes. All treatments were performed in four replicates and, after incubation for 10 weeks, the number of sporophytes generated by each pot was counted. Finally, ten sporophytes were selected from each pot and examined.
Statistical analysis
SAS version 9.3 (SAS Institute Inc., Cary, NC, USA) was used to calculate the mean ± standard error for each treatment, and a factorial analysis was performed using Duncan’s multiple range test, with a significance level of p < 0.05.
Results and Discussion
Effect of medium on gametophyte proliferation
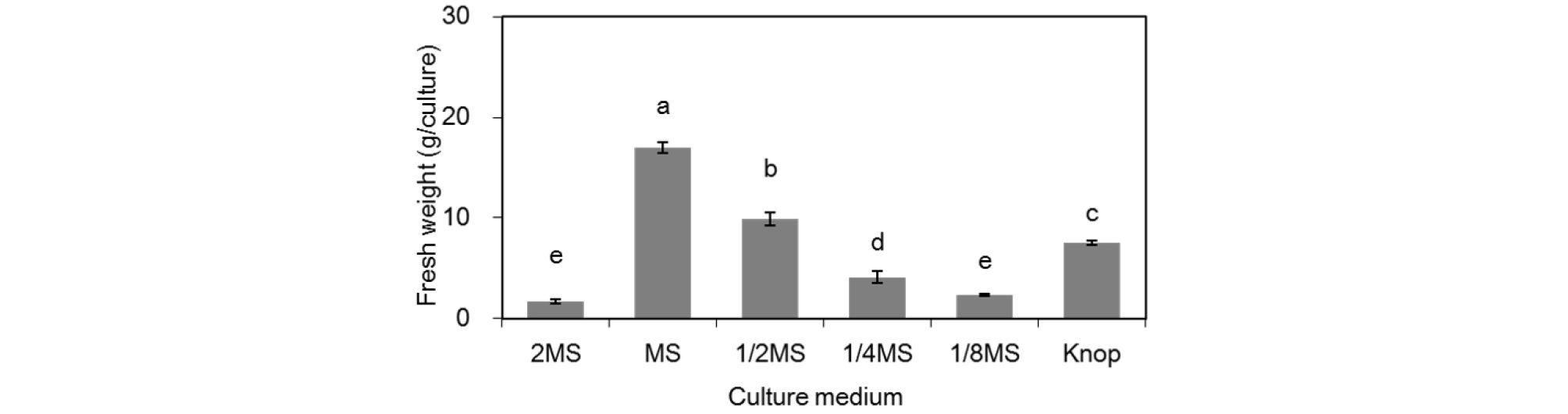
Gametophytes in a uniform state that were obtained from germinated spores were inoculated on several media and incubated for 8 weeks. Gametophyte proliferation was the highest in 1 × MS medium as the fresh weight increased 56.7-fold (Fig. 1). Gametophyte proliferation decreased significantly as the total concentration of MS medium decreased. However, 2 × MS medium, with the highest nutrient content, showed the lowest biomass increase of 5.7-fold. The fresh weight of gametophytes in the Knop medium was 7.5 g, which was greater than that in the 1/4 MS medium.
The gametophytes of ferns, classified as leptosporangiate, grow well in low-nutrient media (Fernández and Revilla, 2003). Among ferns native to Korea, gametophytes of Pteridium aquilinum var. latiusculum show excellent growth and proliferation in medium containing high nutrient content such as 2 × MS medium (Shin et al., 2009). A. aristata shows remarkable proliferation in MS media (Cho et al., 2017). Gametophytes of Microlepia strigosa proliferate actively in 2 × MS and 1 × MS media; however, the proliferation decreases as the total concentration of medium decreases (Cho and Lee, 2017), which is similar to the proliferation of the Asian chain fern in this study. In Osmunda japonica, the growth of gametophytes is active in Knop and 1/8 MS media (Shin and Lee, 2009), which is in contrast to this study.
Therefore, the demand for nutrients for the proliferation of gametophytes does not show a constant trend according to taxa (Shin et al., 2009). For the development of a mass proliferation method for ferns using gametophytes, the selection of an appropriate medium according to species should be considered.
Difference in the development of gametophytes according to medium
In the observation of the morphogenesis of gametophytes according to medium, the mature heart shaped gametophyte with wings developed in 2 × MS, 1 × MS, and Knop media, and cushion was observed only in the 1 × MS medium (Fig. 2). Cushion is a multicellular layer that develops in the center of the gametophyte. It is expressed in dark green plants and is observed in mature gametophytes in which the reproductive organ develops normally (Shorina, 2001). Therefore, the presence of a cushion can be regarded as a standard of healthy gametophytes. In the 1/2, 1/4, and 1/8 MS media, spatula shaped gametophytes, which is the stage preceding heart shaped gametophytes, were observed. In 1/2, 1/4, 1/8 MS, and knop media, gametophytes were thin and some browned areas were observed; thus, this state was considered to be a sign of aging caused by the low content of minerals and carbon in the media (Shin and Lee, 2009).
The gametophyte develops by zygosis of the antheridia and archegonia, which are reproductive organs and develops in the form of hermaphrodite. The in vitro fragmenting and chopping methods that were used in this study enabled the mass propagation of uniform gametophytes, even with a small amount of gametophyte tissue (Miller, 1968). Therefore, to increase the number of normal gametophytes, the development of the reproductive organs, antheridia and archegonia, should be induced. Antheridiogen, a hormone with a skeletal structure similar to that of gibberellin, induces male organ (antheridium) development in juvenile gametophytes (Menéndez et al. 2006). Yamane (1998) reported that addition of exogenous gibberellin induces the formation of antheridium. In a study by Carafa (1990), temperature did not affect the pattern of development of gametophytes on Woodwardia radicans (L.) Sm.; however the sowing density, presence of antheridiogen in the culture medium, and nutritional conditions affected sex expression in the gametophyte.
The concentration of nutrient in medium appears to have a direct effect on ferns gametophyte weight, indicative of its proliferation. The Dryopteris affinis sp. affinis L. exhibited greater prothallus growth when cultured on MS medium (Fernández et al., 1996). Arachniodes aristata (G. Forst.) Tindale prothalli grew most actively in MS medium, but poor growth was showed in rich (2 ×) or lacking (1/2, 1/4, and 1/8 ×) nutrients medium than MS medium (Cho et al., 2017).
In this study, cushion, the developmental background of the reproductive organs, and antheridium were identified in the 1 × MS medium. Therefore, the appropriate medium for the induction of normal development and proliferation of gametophytes in Asian chain ferns was determined to be the 1 × MS medium.
Effect of soil types on sporophyte production
To generate a large number of sporophytes of the Asian chain fern, ground gametophytes were spread on top of the soil mixtures and cultivated for 10 weeks. Sporophyte formation was the greatest in a mixture containing a 1:1:1 (v/v/v) ratio of bed soil, peat moss, and perlite (B1-Pt1-Pr1) or decomposed granite (B1-Pt1-D1), at 782.5 and 708.8 ea/pot, respectively (Table 2). No sporophyte formation occurred with the single use of horticultural soils (B1) and sporophyte formation was achieved at 18.0-68.8 ea/pot in a mixture containing a 2:1 ratio of bed soil and perlite (B2-Pr1) or decomposed granite (B2-D1). The sporophyte formation was at 223.5 ea/pot with the single use of peat moss (Pt1), and the number of sporophytes did not increase when perlite (Pt2-Pr1) or decomposed granite (Pt2-D1) was added to peat moss (v/v), which was different from the trend in bed soil.
Table 2. Effect of soil types on sporophyte formation and biomass of Woodwardia japonica (L.f.) Sm. cultivated for 10 weeks ex vitro
yPot (75 × 75 × 75 ㎜) containing 0.3 L culture medium was used for this experiment.
xMean ± S.E. (n=10) separation within columns by Duncan's multiple range test at P <0.05.
The fresh weight of the sporophyte formed on the aerial part was high in B2-D1 and B1-Pt1-Pr1, and on the underground part was high in B1-Pt1-D1, which was similar to the dry weight analysis (Table 3). The shoots and leaves of sporophytes in B1-Pt1-Pr1 were significantly longer than those in other treatments (Fig. 3). In contrast, sporophyte development did not progress at all because the growth of gametophyte was not confirmed in B1. In addition, a large number of browned sporophytes was observed in B2-Pr1 and B2-D1, which had relatively high ratios of bed soil.
Table 3. Effect of soil types on sporophyte growth of Woodwardia japonica (L.f.) Sm. cultivated for 10 weeks ex vitro
yMean ± S.E. (n=10) separation within columns by Duncan's multiple range test at P < 0.05.
A favorable soil environment for the growth of crops can be created by controlling porosity, air permeability, and water holding capacity (Choi et al., 1997). In native ferns, terrestrial species generally show vigorous sporophyte formation in commercial bed soil, and the mixture soil, which has improved air permeability by adding vermiculite or perlite, is favorable to sporophyte formation in epiphytic species (Lee, 2001). M. strigosa, an epiphytic species, shows excellent sporophyte formation and growth in mixture soil containing at a 1:1:1 (v/v/v) ratio of bed soil, peat moss, and decomposed granite (Cho and Lee, 2017). However, in C. argentea, a small epiphytic fern that grows in rock and stone cracks, the formation of sporophytes is promoted in soils mixed with bed soils and perlite at a 2:1 (v/v) ratio (Jang et al., 2019a); thus, there is a difference in the composition of soils that can promote the formation of sporophytes among species of epiphytic fern. In Cho et al. (2017), gametophytes of A. aristata do not develop to the sporophyte in the peat moss. In P. braunii, the most sporophytes were formed in mixture soil with bed soil and granite at a 2:1 (v/v) ratio (Kwon et al., 2017).
The Asian chain fern is an epiphytic species that grows on rocks and trees (BK, 2019). Therefore, the mixture of bed soil, peat moss, and perlite in a 1:1:1 ratio (v/v/v) led to the accelerated formation and growth of sporophytes in this study. According to an analysis by Jang et al. (2019a), in the same bed soil and peat moss as used in this study accounts for more than 80% of the water phase. Therefore, the water phase content must be adjusted for the production of Asian chain fern using bed soil and peat moss. However, perlite is standardized and cheaper than the same volume of decomposed granite; thus, perlite is more suitable for the economical production of sporophytes.
In optimal soils, sporophytes of the Asian chain fern had a greater production (782.5 ea/pot) from the same input of gametophytes than that in M. strigose (44.1 ea/pot, Cho and Lee, 2017) and C. argentea (74.3 ea/pot, Jang et al., 2019a); thus, the production efficiency was high in the Asian chain fern. Consequently, the soil mixture containing a 1:1:1 (v/v/v) ratio of bed soil, peat moss, and perlite (B1-Pt1-Pr1) was suitable for the proliferation and development of gametophytes and sporophytes by the maintenance of adequate water holding capacity and air permeability. The results of this study will contribute to farm household income by enabling the mass production of uniform seedlings of the Asian chain fern, which are valuable as ornamental horticultural crops.