Introduction
Materials and Methods
Plant material
Surface-sterilization of plant materials
In vitro microropagation and examination of microorganisms
Results and Discussion
Establishment of suitable surface-sterilization in Korean native lily species
Examination of the non-contamination in adventitious bulbs
Introduction
Plant genetic resources are important for agriculture, food security and agro-biodiversity as valuable genetic materials and potential value (Kaviani, 2011), and are generally conserved in genebanks or field as seed and tissue samples and in the wild areas, which is they grown (Matsumoto, 2017). Plant genetic resources conservation has become increasingly important as more plants have become threatened or rare.
Lily, a member of the genus Lilium, is globally one of the most economically important ornamental crops used for flowers in many countries (Xia et al., 2006). There are many lilies germplasm and approximately 15 native Lilium species are distributed in Korea (Kim, 2008). Most native lilies have specific characteristics such as flower shapes, colors and disease-resistance, hence the indigenous lily species has considerable competitiveness and has very high development value. The genus Lilium is facing a serious threat of genetic erosion due to weak immune system against pathogens and pests, and climate change. It is not always possible to preserve under natural conditions. Preservation in field collections is labor intensive and there is the risk of loss from diseases, adverse weather and other factors. In vitro tissue culture conservation is susceptible to contamination and somaclonal variation (Chen et al., 2011). Cryopreservation is one of the ideal and suitable methods for long-term storage of plant germplasm resources (Wang and Perl, 2006; Yi et al., 2018). In the past several decades, several storage strategies for cryopreservation have been developed, such as droplet- vitrification method (Kim et al., 2010; Leunufna and Keller, 2003) and encapsulation-vitrification method (Hirai and Sakai, 1999). For cryopreservation of plant genetic resources, first of all, it is important to acquire the disease-free tissues before cryopreservation. The plant contaminated with diseases and pathogens are decreased the multiplication, survival and regeneration rate, and high quality of plant genetic resources after cryopreservation (Wang and Valkonen, 2009). In vitro culture of Korean native lilies has been tried the factors affecting regeneration ability, multiplication and growth in L. lancifolium, L. callosum, and L. hansonii (Goo et al., 2004; Kim et al., 1995; Paek and Shin, 1983; Park et al., 1998). Also, for the preservation of long-term low temperature, the lilies have been cryopreserved by encapsulation- dehydration, vitrification and droplet-vitrification in several previous studies (Bouman et al., 2003; Chen et al., 2007; Chen et al., 2011; Matsumoto et al., 1995; Zhang et al., 2004). Nevertheless, many researchers are still trying to increase the lilies regrowth rate or apply it to a wider range of lily native species and cultivars. Establishment of efficient plant regeneration for cryopreservation is a basic requirement. From valuable preservation point of genetic resource of view, disease-free propagation method would provide to be the fastest for the increasing of regeneration rate for Lilium cryopreservation. Until now, there are very fewer studies that have been focused on in vitro micropropagation for the production of disease-free propagation in Korean native species (Jin et al., 2001). We tried to examine in vitro micropropagation of two Korean native species (L. hansonii and L. tsingtauense) belonging to section Martagon for the establishment of effective axenic cultures of Lilium.
Materials and Methods
Plant material
The lily accessions used in this study are Lilium tsingtauense and Liliumhansonii belonging to Liliaceae and are given in Table 1. The lily accessions were obtained from the National Agrobiodiversity Center of the Rural Development Administration (RDA).
Table 1. Effect of sterilants on percent contamination, necrosis, and survival of inner scales of Lilium on the MS medium supplemented with PPM in five accessions of two Korean native lilies species
Surface-sterilization of plant materials
The plant materials were used three Lilium tsingtauense and two L. hansonii accessions contaminated with microorganisms to obtain the disease-free bulblets from scales for cryopreservation in Lilium. Bulb inner scales of these five accessions were sterilized to the modified surface-sterilization method according to previously described by Askari et al. (2014). Bulbs contaminated with microorganisms were washed to remove bacterial clump and residue under running tap water. Scales detached from the bulbs were surface-sterilized for 20 min in 1.6% (w/v) sodium hypochlorite (NaClO). And then, these scales rinsed for 3 min with sterile distilled water and 3 min with 0.03% NaClO. In the last process of rinsing scales after surface-sterilization, we submerged and sterilized the tissue by rinsing in 0.03% NaClO for 2 hours instead of sterile distilled water. The inner scales were dried on the sterilized paper for 3 min.
In vitro microropagation and examination of microorganisms
Whole stages during In vitro microropagation were placed in the incubation room at 25°C under 16 hours photoperiod. The inner scales were inverted and cultured on Murashige & Skoog (MS) medium supplemented with 0.1% Plant Preservative Mixture (PPM, Apollo Scientific Limited, UK), which is a robust broad-spectrum biocide made for use in plant tissue culture (Fig. 1C) (Guri and Patel, 1998). PPM targets bacteria and fungi in plant tissue culture growth media as well as contaminated tissue. The adventitious bulbs induced from inner scales were transferred to MS medium, immediately. We scored the survival rate and non-contamination after 2 weeks and 3 weeks in culture, respectively. Microorganisms were inoculated on Brain Heart infusion agar (BHIA, Difco, Becton Dickinson) medium by streaking it over the surface with an inoculating needle in incubator (Lab Companion, IL-11-4C, Korea) at 30°C for 3 weeks (Fig. 2B). We checked either bacterial incidences or not on the BHI agar medium plates, and then non-contaminated bulblets were transferred to MS medium free hormones and checked the presence of microorganisms after 3 weeks.
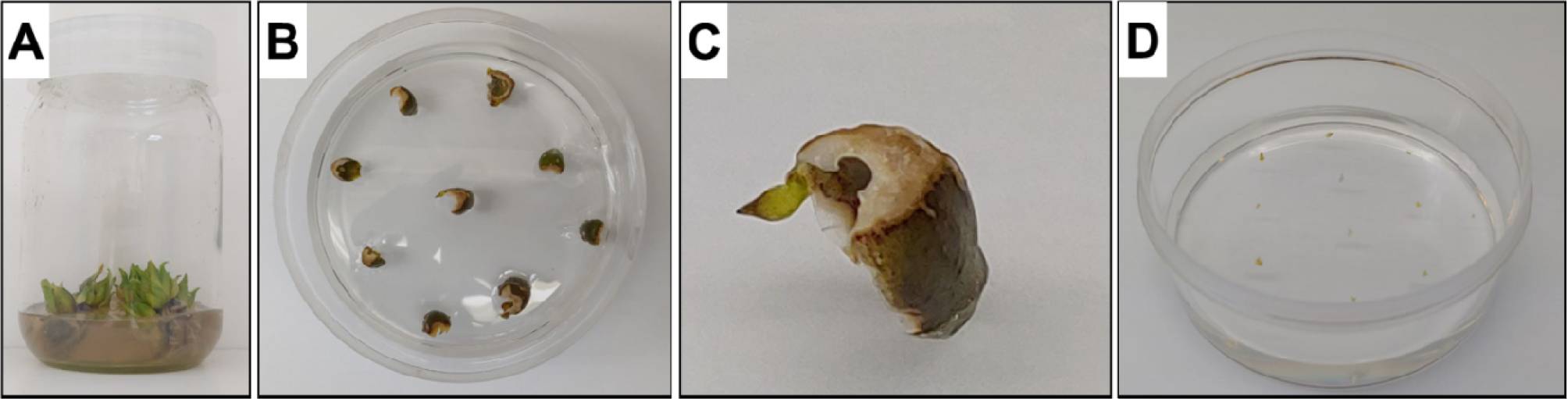
Fig. 1.
Procedure of Lilium bulblets induction from inner scales. A; contaminated Lilium bulbs, B; Lilium bulb inner scales cultivation treated with 1.6% NaOCl and rinsed with 0.03% NaOCl for 2 hours on MS medium containing PPM, C; formation of the first bulblet, D; induced bulblets were transferred to MS medium free hormones.
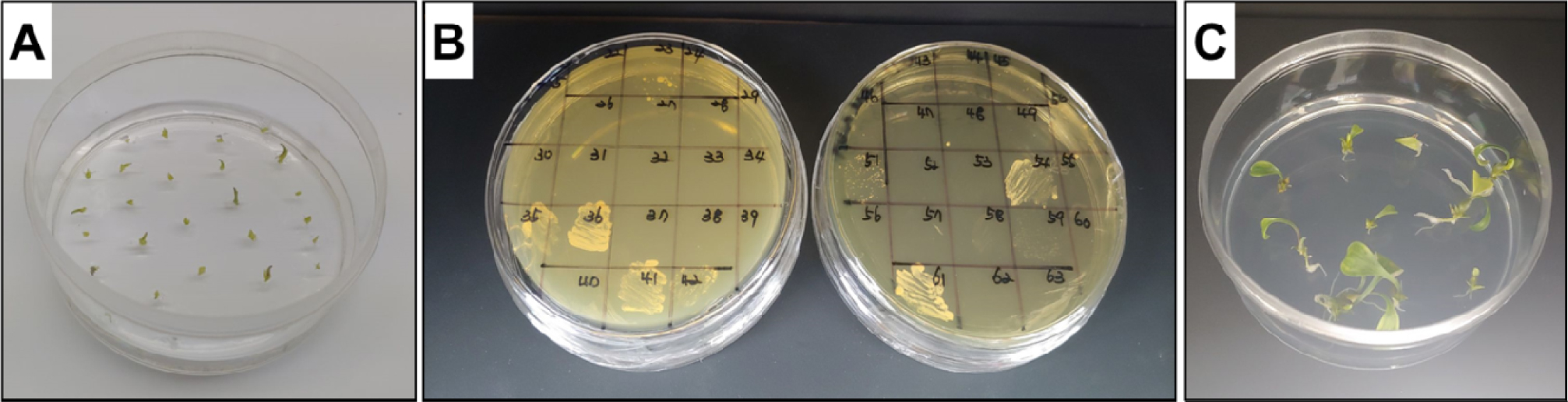
Fig. 2.
The presence of microorganisms was examined in the newly induced bulblets. A; transferred new bulblets on MS medium free hormones, B; contamination of microorganisms was examined with BHI agar medium, C; Non-contaminated bulblets transferred to MS medium free hormones and checked the presence of microorganisms for 3 weeks.
Results and Discussion
Establishment of suitable surface-sterilization in Korean native lily species
To achieve suitable sterilizing of explants and successful culture in Korean native lily species, Lilium explants, inner bulb scales, were exposed to 0.03% NaClO rinsing water for 2 hours instead of sterile distilled water in the last process of rinsing before plating on the MS medium including PPM (Fig. 1B). The results showed that L. tsingtauense accessions were observed considerable variations, which ranged from 53.9 to 100% with a mean value of 76.8% in three species and two L. hansonii accessions were checked from 84.5 to 85.5% with a mean of 85% survival rate, respectively (Table 1). During the rinsing after surface-sterilization, the sterile distilled water becomes contaminated with microorganisms lived within the explants (Thakur and Sood, 2006). Contamination of microorganisms that had not been killed during surface-sterilization was controlled by 0.03% NaClO rinsing water (Askari et al., 2014). The application of PPM in vitro culture is able to reduce contamination and prevent the growth of microorganism (Guri and Patel, 1998; Niedz, 1998). Due to these reasons, we have transferred the adventitious bulbs to MS medium free hormones to choose the disease-free bulblets (Figs. 1D, 2A).
Examination of the non-contamination in adventitious bulbs
To examine either bacterial incidences or not, all the adventitious bulbs grown on MS medium were checked the presence of microorganisms on the BHI agar medium plates for 3 weeks (Fig. 2B). The non-contamination rate of Korean native lilies based on newly induced bulblets showed in the Fig. 2 and Table 2. The results indicated that the non- contamination rate were shown ranged from 75.0 to 94.1% with mean value of 83.2% in L. tsingtauense species, and that L. hansonii were observed 85.1 to 91.7% with mean value of 88.4% (Table 2). It can be suggested from our results that application of both the 0.03 % NaClO rinsing water and MS medium containing PPM combination were removed contamination of bulblets formation from contaminated lilies bulbs.
Table 2. Contamination, necrosis and survival rate of small bulbs induced from each of the survival inner scales after transfer to the MS medium
The indigenous plants of Lilium have been valued as a source of traits that can be used in breeding programs and to improve the disease resistance and the quality of new varieties (Hwang et al., 2015). Therefore, it is very important to acquire conditions of explants for the bulblet formation by the scale in vitro micropropagation, and to orientate effective strategies of long-term preservation in several Korean native lilies. However, very little is known about the strategies of cryopreservation using explants among Korean species of Lilium. We conducted to get the disease-free bulblets of Korean native lily species by using contaminated lilies on in vitro micropropagation. Based on these results, this study will help to reduce or eliminate the contamination of microorganisms, and that produces the disease-free bulblet. In addition, the selected and proliferated accessions in our study will be used to increase the regeneration and survival rate in the lilies cryopreservation program for the long-term conservation of Korean native lilies species.




