Introduction
Materials and Methods
Plant materials and culture media
Media, culture condition and shoot regeneration frequency
Treatment of plant growth regulators for shoot multiplication
Pretreatment of BA and selection of explant types
Histological observation on shoot induction
Results and Discussion
Shoot induction by explant types
The optimal concentration of plant growth regulators (PGRs) for shoot formation
Effect of pretreatment and non-pretreatment of BA into explants on shoot induction
Histological study on shoot formation
Introduction
Soybean [Glycine max (L.) Merr.] is well known as a recalcitrant plant in in vitro regeneration. It plays a potential in worldwide for human and animal feed as well as vegetable oil source. Five Korean soybean cultivars, Dawon, Pungsan, Daewon, Taekwang and Chongdoo 1 were one of recommended in Korean elite cultivars. Regeneration by organogenesis is a critical step for plant tissue propagation. It has been reported that regeneration efficiency is affected by genotype, explant source, age and size of explant, plant growth regulators (concentration and kind of plant growth regulators), basal medium content and culture conditions (light and temperature) (Raza et al., 2017; Franklin et al., 2004; Sairam et al., 2003; Yildiz et al., 2002; Bailey et al., 1993). In legume plants, many previous researches have been used different explants for shoot regeneration (Zhang et al., 2014; Hong et al., 2006; Yan et al., 2000; Franklin et al., 1993; Yue-Sheng et al., 1990). MS medium (Murashige and Skoog, 1962) is the most frequently used in plant tissue culture while B5 medium is commonly used in some approaches or shoot induction. Mariashibu et al. (2013) indicated that nutrition requirement as a medium composition plays an important role for plant regeneration. Cytokinins are generally used for shoot induction. One of them is benzyladenine (BA) which is commonly used either alone or in the combination with a low concentration of other cytokinins; kinetin/BA or thidiazuron (Choi et al., 2019; Ma and Wu, 2008; Franklin et al., 2004). Many studies have been reported that MS media supplemented with BA 2 to 3 ㎎/L promoted multiple shoot formation from cotyledonary node (Kim et al., 2016; Ma and Wu, 2008; Jackson and Hobbs, 1990).
Furthermore, histological study is important in in vitro plant regeneration in order to figure out the origins of the plant during organogenesis (Dibax et al., 2013). However, few studies have been researched on the connection between histological and kinds of plant growth regulators during shoot regeneration (Wang et al., 2015). The observations were executed to understand the process of adventitious shoot formation during in vitro morphogeneis from explants.
In this study, we investigated the optimum types of explant, and the optimum concentration and kinds of plant growth regulator (PGR) for plant regeneration in five Korean soybean cultivars. Also, we measured that the pretreatment of BA onto cotyledonary node explant before in vitro culture affect shoot regeneration. For understanding the organogenesis process morphologically that is originated from the region of the cotyledonary node in legumes, the histological changes were investigated by using the cotyledonary node explants in a Korean elite soybean cultivar.
Materials and Methods
Plant materials and culture media
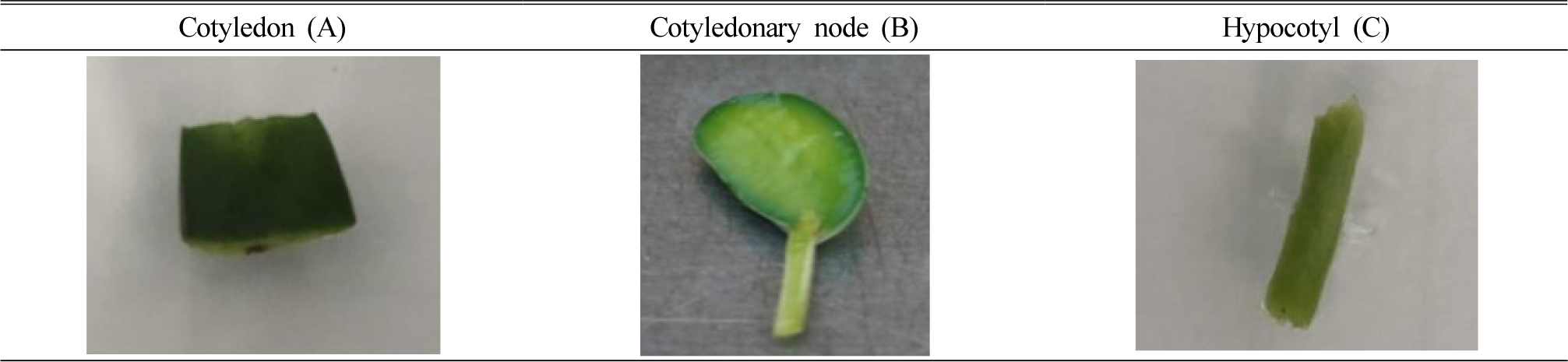
Five Korean elite soybean cultivars (Dawon, Pungsan, Daewon, Taekwang and Chongdoo 1) were used for plant materials in this experiment. After the surface sterilization with ethanol 70%, 30s to 1 min approximately, dry seeds were soaked 1% sodium hypochlorite solution for 15 minutes followed by washing with autoclaved distilled water. After germination, cotyledon, cotyledonary node and hypocotyl of 7-10 days grown in vitro seedling (Fig. 1), were used as explant. Plant segments were cultured on shoot induction media, MS basal medium supplemented with B5 vitamin with concentration of 6-benzyladenine (BA) at 2 ㎎/L (MSBA2) which was suggested by the previous studied (Kim et al., 2016).
Media, culture condition and shoot regeneration frequency
MS media supplemented with B5 vitamin were used as basal media for organogenesis (Raveendar et al., 2009). And the MS medium was reinforced with 30 g/L sucrose, 8 g/L agar and the pH of the medium was regulated to 5.7 ± 0.5 after adding plant growth regulators. The medium was autoclaved at 121℃, for 20 min and poured in a culture plates. All the culture media were kept in a culture room at 26 ± 2℃ under 16-h photoperiod provided by cool-white fluorescent lamps at 25 μmol/㎡/sec.
To estimate the optimal shooting condition, basal medium was supplemented with single of BA or kinetin with concentration (0, 1, 2, 4 ㎎/L) and the combinations of BA and kinetin (1, 2 ㎎/L).
The number of shoots and the shoot regeneration frequency were recorded after 21 days in culture. The frequency of shoot regeneration was calculated as follows:
Treatment of plant growth regulators for shoot multiplication
Regeneration via direct organogenesis was investigated in the five soybean cultivars, ‘Dawon’, ‘Pungsan’, ‘Daewon’, ‘Taekwang’ and ‘Chongdoo 1’. After germination 7 to 10 days (when cotyledon turns green), cotyledonary nodes of the five soybean cultivars were used as explant. The segments (about 1 ㎝ in length) of soybean was transferred to shoot induction media containing MS medium supplemented with B5 vitamin as a basal media, supplemented with various plant growth regulators at different concentration including BA (0, 1, 2, 4 ㎎/L), kinetin (0, 1, 2, 4 ㎎/L), a combination BA and kinetin. The frequency of shoot regeneration was calculated as mention above.
Pretreatment of BA and selection of explant types
Two types of cotyledonary node explants of the five soybean cultivars including Glycine max L. cv. ‘Dawon’ were prepared by a scalpel to remove any excess hypocotyl remaining 0.5 ㎝ in length (Fig. 2-A) and half-cotyledons were split off (Fig. 2-B). All the explants were dipped in 200 ㎎/L of BA solution for 1 min pretreatment and then in vitro cultured on shoot induction media supplemented with 2 ㎎/L BA. After 3 weeks, the shoot number and the shoot regeneration ratio were measured with three replications.
Histological observation on shoot induction
Cotyledonary node explants of Glycine max L. cv. ‘Dawon’ were in vitro cultured on the shoot induction MS media containing B5 vitamin supplemented with 2 ㎎/L BA. The cotyledonary node specimens were selected in 0, 3, 6, 9, 12, 15, 18 and 21 days, and fixed in a mixture of ethanol, formaldehyde and acetic acid for 48 hours. Then, the specimens were trimmed and dehydrated by immersing the specimens in a series concentration of alcohol (70~100%) to remove the water and the formalin from the specimens. After then the specimens were infiltrated and embedded into glycol methacrylate (JB-4 Embedding Kit; Poly Sciences, Warrington, PA, USA). Serial sections, in 5 ㎛ thick, were cut by an ultra-microtome (MT-990 Type S, RMC Boeckeler, AZ, USA), mounted on slides, and stained by periodic acid-Schiff reaction method using hematoxylin (Sigma Co.) as a counter- strain. After rinse slides with tap water, kept the slides warm until dried on a hot plate at 35℃ for 10 minutes. The slides were mounted coverslip onto the section on slide glass with Permount (Fischer Chemical, Pittsburgh, PA, USA) in a week. Finally, the histological investigation of shoot differtiation was observed in a light microscope (Nikon E200, Nikon, Tokyo, Japan).
Results and Discussion
Shoot induction by explant types
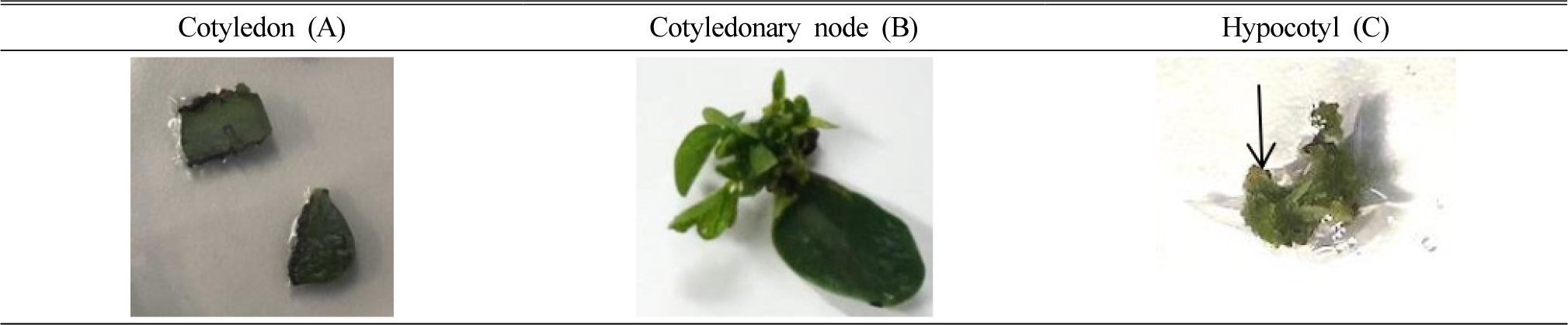
In order to check optimized explant type, three kinds of explants including cotyledon, cotyledonary node and hypocotyl of soybean cultivar ‘Dawon’ were cultured on shoot induction media for 21 days as shown in Fig. 1. Only the cotyledonary node segments gave a response for shoot induction (Fig. 3-B). While both of cotyledon (Fig. 3-A) and hypocotyl (Fig. 3-C) segments did not showed the efficient shoot regeneration, but calli were produced at the surface of cutting region of the explant in the hypocotyl segment (Fig. 3-C, arrow point). Many previous researchers have been reported that different explants were used for shoot formation in in vitro culture of legume plants. A whole cotyledonary node of soybean has been used as an explant (Zhang et al., 2014), while the other tissue types such as young leaves (Yue-Sheng et al., 1990), hypocotyl (Franklin et al., 1993), immature embryo (Yan et al., 2000), axillary bud (Hong et al., 2006) have been used. The cotyledonary node explant was the best explant in this experiments and the result was consistent with the previous reports (Kim et al., 2016; Sairam et al., 2003).
The optimal concentration of plant growth regulators (PGRs) for shoot formation
The cotyledonary node explants yielded shoots on shoot formation frequency with 66.67-100% when treated with BA or kinetin or a combination of BA-kinetin (Table 1). Media containing 2.0 ㎎/L of BA showed the best frequency for multiple shoot induction. The highest average number of shoots/explant was 2.12 in soybean cultivar ‘Pungsan’, followed by ‘Dawon’ (1.48 shoots/explant), ‘Taekwang’ (1.32 shoots/explant), ‘Chongdoo 1’ (1.30 shoots/explant) and ‘Daewon’ (1.25 shoots/explant), respectively. The range of shoot numbers varied from 1 to 9 shoots in soybean cultivar ‘Pungsan’, while that of ‘Dawon’ was varied from 1 to 4 shoots. In addition, individual explant showed different response on shoot regeneration ability, even in the same culture conditions. The synergistic effect by a combination of BA-kinetin was not shown in our results, while Franklin et al. (2004) reported a synergistic effect in the presence of BA and TDZ. Moreover, genotype was an important factor in terms of the in vitro regeneration efficiency (Raza et al., 2017; Kim et al., 2016; Franklin et al., 2004; Sairam et al., 2003; Bailey et al., 1993).
Table 1. Shoot induction from cotyledonary node explant of Glycine max L. 5 cultivars on shoot induction media supplemented with different concentration and kinds of plant growth regulators after 3 weeks
yA-E: Soybean cultivars follow by A: Dawon, B: Pungsan, C: Daewon, D: Taekwang, E: Chongdoo 1.
xMeans with the same letters within the column are not significantly different by DMRT (p ˂ 0.05). ns: not significant.
wNumbers in the parentheses mean (A/B) = Number of explants regenerated in to shoots (A) per total number of cultured explants (B).
Effect of pretreatment and non-pretreatment of BA into explants on shoot induction
As shown in Table 2, pretreatment by a high concentration of BA (200 ㎎/L) before in vitro culture onto the explants accelerated the shoot number per explants compared with non-pretreated control. Both of half-split cotyledonary node and cotyledonary node explants showed high frequency of shoots. In the pretreatment condition, the percentage of shoot induction ranged on 88-97% with the maximum 97.09% in half-split cotyledonary node of soybean cultivar ‘Dawon’. While in the non-pretreatment, the percentage of shoot induction ranged on 83-92%. By the pretreatment of BA onto both half-split cotyledonary node and cotyledonary node explants, ‘Dawon’ achieved the highest number of shoots per explant followed by the soybean cultivar ‘Pungsan’, ‘Taekwang’, ‘Daewon’ and ‘Chongdoo 1’, respectively.
Table 2. Effect of pretreatment with high concentration of BA on percentage of shoot induction in half-split cotyledonary node and cotyledonary node explants after 21 days cultures in soybean cultivars (Glycine max L.)z
yPT: Pretreatment.
xNT: Non-pretreatment.
The pretreatment of explants with plant growth regulators is the general technique to improve the induction of shoot (Thomas, 2007). In our experiment, pretreatment by the BA (200 ㎎/L) on the surface of half-split cotyledonary node and cotyledonary node explants promoted shoot regeneration compared with non-pretreated tissues. In cotyledonary node of cowpea, pretreatment with 3 ㎎/L BA gave the best response in terms of shoot number and shoot length (Tie et al., 2013). In this experiment, half-split cotyledonary node showed the best number of shoot, while percentage of shoot induction was not significantly different. However, Compton and Gray (1993) indicated that explant cutting types could influence the shoot formation and cotyledon based explant of watermelon showed higher percentage of explant producing shoot compared with cotyledon cut in half longitudinally.
Histological study on shoot formation
Our histological investigation gave evidence for direct formation of organogenesis structures at cotyledonary node region in soybean (Fig. 4). The results correlated with the cell formation and differentiation that occurred during shoot regeneration. During 3-6 days of culture on shoot induction media containing BA 2 ㎎/L, the meristematic tissue around cotyledonary node was dividing in both of ‘Dawon’ (Fig. 4) and ‘Pungsan’ (not shown data) cultivars. Epidermis and sub-epidermis cells (cortical cells layers) became competent and dedifferentiated from parenchyma cells to compact globular meristemoids. Meristemoids were formed both anticlinal and periclinal division to the plane. Cell initiated from outer epidermal cells and cortical cells and then forms afterward. During 9 to 21 days vascular cells expanded, and ground tissues were gradually differentiated by changed to thin wall cells. Shoot bud had usual dome-shaped meristems with two lateral leaf-primordia which are connected with the vascular system. Main vascular tissue of mother plant was expanded to link with pro-vascular (new shoot) cambium. For shoot differentiation more time is needed in soybean shoot formation than that of pea (Kantayos, 2019; Kantayos and Bae, 2019).
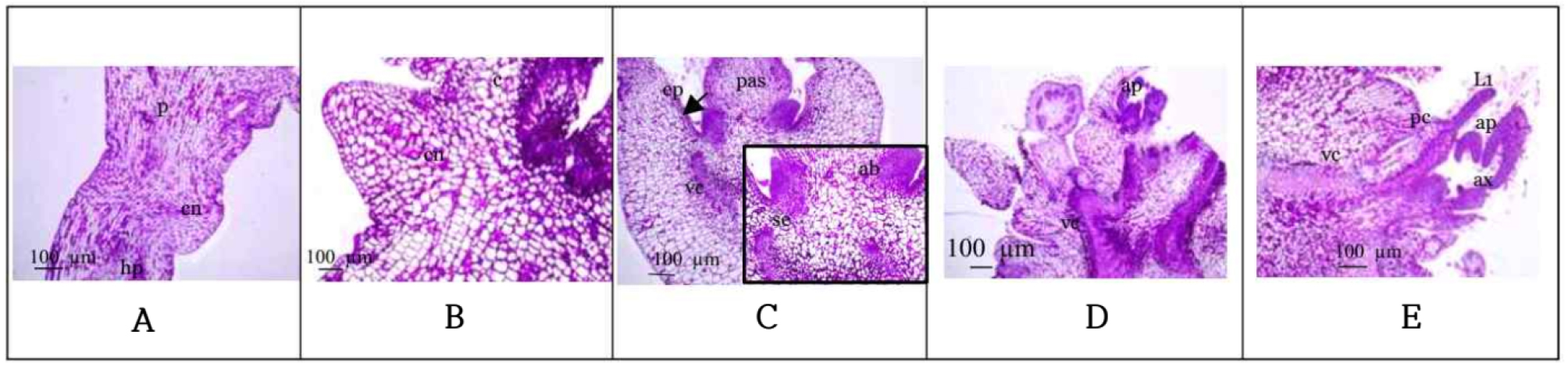
Fig. 4.
Histological analysis of the shoot induction process in Glycine max L. cv. ‘Dawon’. The cotyledonary nodes were cultured for 0 to 21 days on the basal MS with B5 vitamin and 2 ㎎/L BA. A: 0 day, B: 3-day, C: 6-day, D: 12-day, E: 21-day. Abbreviations; ab: axillary bud, ap: apical meristem, ax: axillary meristem, c: cotyledon area, cn: cotyledonary node, ep: epidermal cells, hp: hypocotyl area, L1: leaf primordia, p: parenchyma, pas: primary axillary shoot, pc: procambium, se: sub-epidermal cell, vc: vascular cambium.
In conclusion, cotyledonary node explant of the soybean cultivar was selected and used as explant for estimating the optimal shoot differentiation conditions. MS as a basal media supplemented with B5 vitamin were used as shoot induction media. Also, the optimum PGR condition for the cultivar ‘Dawon’ was BA 2 ㎎/L. Even though cut cotyledon did not show any shoot induction response at wounding point, both of half-split cotyledonary node and cotyledonary node showed the multiple shooting. Especially, the pretreatment of BA onto cotyledonary node explants be in vitro culture, promoted shoot induction in 21 days culture.