Introduction
Materials and Methods
Experimental materials
Sample extraction
DPPH radical scavenging activity
ABTS radical scavenging activity
Reducing power
Quantification of acteoside by HPLC-PDA analysis
Cell culture
Immunoblotting
Revers transcription-Polymerase chain reaction
Statistical analysis
Results and Discussion
Quantification of acteoside
Antioxidant activity
Whitening activity
Introduction
The skin, which separates the epidermis from the dermis, mainly functions to protect the body from physical or chemical stimulation and prevents disproportionate moisture loss (Chung et al., 2003; Fisher et al., 2002; Kim et al., 2014). Skin aging is the cumulative result of oxidative damage to cutaneous tissue (Wickens, 2001), and is caused by intrinsic and extrinsic factors, which include exposure to ultraviolet radiation, causing photoaging (Chung et al., 2003; Seo et al., 2001). Melanin is synthesized in the melanocytes through the process of melanogenesis, in response to extrinsic aging factors (Tsatmali et al., 2002). Reactive oxidant species (ROS) are known to cause skin aging as well as other human diseases (Luft, 1994), and oxidative stress can lead to an abnormal redox state of cell membrane proteins in skin cells, resulting in melanogenesis (Kim and Uyama, 2005). Under normal conditions melanin has a beneficial effect on the photo-protection of human skin (Costin and Hearing, 2007). However, an excessive accumulation of melanin causes dermatological diseases such as freckles, solar lentigo (age spots), and melanocarcinoma (Ahn et al., 2006, Brenner and Hearing, 2008; Iozumi et al., 1993; Li et al., 2003; Unver et al., 2006). Melanin synthesis is similar to the oxidative stress caused by ROS. Therefore, antioxidant activity is supposed to suppress melanogenesis (Eberlein-Konig et al., 1998). Melanogenesis regulates tyrosinase, tyrosinase-related protein 1 (TRP-1) and tyrosinase-related protein 2 (TRP-2), which are modulated by microphthalmia-associated transcription factor (MITF) in melanogenesis (Ahn et al., 2008). Thus, the downregulation of tyrosinase, TRP-1, TRP-2, MITF activity has been proposed to be responsible for decreasing melanin production (Curto et al., 1999). Abeliophyllum distichum Nakai is a deciduous shrub of in the family Oleaceae, and is regarded as a valuable plant resource because there is only one species in the world (Park, 2011). We evaluated the antioxidant and whitening activity of extracts by water and prethanol A to develop A. distichum as a safe and effective material for cosmetic use.
Materials and Methods
Experimental materials
A. distichum Nakai (voucher number: JWU-031) was collected from Misun-hyang Theme park, Seongbul-Mountain Recreation Forest, 78, Chungmin-rogigok-gil, Goesan-eup, Goesan-gun, Chungcheongbuk-do, Korea. Acetonitrile, chloroform, dimethyl sulfoxide, ethyl acetate, methanol, petroleum ether and prethanol A (HPLC-grade) were purchased from Merck (Darmstadt, Germany). Dulbecco’s modified Eagle’s medium (DMEM), 10% (v/v) fetal bovine serum, penicillin/streptomycin, and trypsin were purchased from Hyclone (Logan, UT, USA). The primary and secondary antibodies were purchased from Abcam (Cambridge, UK) and Santa Cruz Biotechnology (Dallas, TX, USA). All electrophoresis chemicals were purchased from Bio-Rad Labs (Hercules, CA, USA). All chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA), unless otherwise specified. Standard acteoside was purchased from Sigma-Aldrich.
Sample extraction
A. distichum was extracted with 100% water and 50, 70, 100% prethanol A using a autoclave (121℃, 1.2 atm) for 15 min. The prethanol A extracts were filtered and concentrated by using a vacuum evaporator (N-1110S, EYELA, Shanghai, China). The water extracts were freeze drying and stored in a refrigerator until use.
DPPH radical scavenging activity
DPPH radical scavenging activity was measured according to Bondet method (Bondet et al., 1997) with some modifications. DPPH solution containing 1,1-diphenyl-2-picryl hydrazyl (DPPH) in ethanol was prepared. Sample and DPPH solution were mixed and incubated for 20 min in the dark at room temperature. Absorbance was measured using a UV/Visible spectrophotometer (Xma-3000PC, HumanCorp, Seoul, Korea) at 515 ㎚. DPPH radical scavenging activity was calculated according to the following equation:
DPPH radical scavenging activity (%) = [1 − (ASample − ABlank)/ACcontrol] × 100
ASample=Absorbance values of DPPH radicals after treatment with sample.
ABlank=Absorbance values of DPPH radicals with ethanol.
AControl=Absorbance values of DPPH radicals.
ABTS radical scavenging activity
ABTS radical scavenging activity was measured as described by Van den Berg et al. (1999) with some modifications. ABTS solutions containing 2,2'-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt and potassium persulfate in distilled water were prepared 24 h prior to the experiment. Sample and ABTS solution were mixed and incubated for 20 min in the dark at room temperature. Absorbance was measured using a UV/Visible spectrophotometer at 732 ㎚. ABTS radical scavenging activity was calculated according to the following equation:
ABTS radical scavenging activity (%) = [1 − (ASample − ABlank)/ACcontrol] × 100
ASample=Absorbance values of ABTS radicals after treatment with sample.
ABlank=Absorbance values of ABTS radicals with ethanol.
AControl=Absorbance values of ABTS radicals.
Reducing power
Reducing power was measured according to Oyaizu method (Oyaizu, 1986) described with some modifications. The mixture, sample, potassium phosphate buffer (pH 6.6) and potassium hexacyanoferrate (Ⅲ), was reacted at 50℃ for 20 min. After that, it was cooled and added to trichloroacetic acid (TCA). The mixture was centrifuged at 2000 × g for 5 min. Ferric chloride was mixed to the supernatant properly. Absorbance was measured using a UV/Visible spectrophotometer at 700 ㎚. Reducing power was calculated according to the following equation:
Reducing power (Relative value of Acontrol, %) = [1 − (ASample − ABlank)/Acontrol] × 100
‘ASample’=Absorbance values of Reducing power after treatment with sample.
‘ABlank’=Absorbance values of Reducing power with ethanol.
‘Acontrol’=Absorbance values of Reducing power after treatment with positive control (L-ascorbic acid).
Quantification of acteoside by HPLC-PDA analysis
A Waters 2695 system (Milford, MA, USA) equipped with Waters 2996 Photodiode array detector (PDA) was used to analyze the prethanol A and water extracts from A. distichum and the standard acteoside. Separation was carried out on an Xbridge-C18 (250 × 4.6 ㎜, 5 ㎛) with a C18 guard column. The binary mobile phase consisted of acetonitrile (solvent A) and water containing 1% acetic acid (solvent B). All solvents were filtered through a 0.45 ㎛ filter prior to use. The flow-rate was kept constant at 1.0 ㎖/min for a total run time of 20 min. The system was run with a gradient program: 0–20 min: 90% B to 50% B. The sample injection volume was 10 ㎕. Peaks of interest were monitored at 200–400 ㎚ by a PDA detector and compared with the standard acteoside.
Cell culture
B16 F10 cells were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA) and grown in DMEM supplemented with 10% (v/v) fetal bovine serum, 100 U/㎖ penicillin, and 100 ㎍/㎖ streptomycin. The cells were maintained at 37°C under a humidified atmosphere of 5% CO2.
Immunoblotting
B16 F10 cells were cultured in 6-well plates at 37°C in an incubator with a humidified atmosphere of 5% CO2. The cells were washed with 1 × phosphate-buffered saline and lysed in radio immuno precipitation assay buffer (Thermo Scientific, Waltham, MA, USA) supplemented with protease inhibitor cocktail (Sigma-Aldrich), and then centrifuged at 12,000 × g for 15 min at 4°C. The protein concentration of the sample was determined by the Bradford protein assay (Bio-Rad, Hercules, CA, USA). The proteins were mixed with Laemmli buffer and boiled at 95℃ for 5 min. The proteins were separated by 10% SDS-PAGE and transferred to a polyvinylidene fluoride membrane (Bio-Rad). The membranes were blocked for non-specific binding with 5% nonfat dry milk in Tris-buffered saline containing 1% Tween 20 (TBS-T) for 30 min at room temperature and then incubated with specific primary antibodies in 3% nonfat dry milk at 4℃ overnight. After washing three times with TBS-T, the blots were incubated with horseradish peroxidase-conjugated immunoglobulin G for 1 h at room temperature and chemiluminescence was detected using ECL Western blotting substrate (Bio-Rad) and visualized with FluorChem E (Cell Biosciences).
Revers transcription-Polymerase chain reaction
Total RNA was prepared from B16 F10 cells using a NucleoSpin® RNA Plus (Macherey-Nagel, Düren, Germany) and total RNA was synthesized using ReverTra Ace-α- (Toyobo, Osaka, Japan) according to the manufacturer’s protocol for cDNA synthesis. PCR was carried out using Quick Taq® HS DyeMix (Toyobo) with primers for Tyrosinase forward 5’-GAG AAG CGA GTC TTG ATT AG-3’, reverse 5’-TGG TGC TTC ATG GGC AAA ATC-3’, TRP-1 forward 5’-GCT GCA GGA GCC TTC TTT CTC-3’, reverse 5’-AAG ACG CTG CAC TGC TGG TCT-3’, TRP-2 forward 5’-CCT GTC TCT CCA GAA GTT TG-3’, reverse 5’-CGT CTG TAA AAG AGT GGA GG-3’, MITF forward 5’-AGC GTG TAT TTT CCC CAC AG-3’, reverse 5’-TAG CTC CTT AAT GCG GTC GT-3’, GADPH forward 5'-AAC TTT GGC 223 ATT GTG GAA GG-3', reverse 5'-ATG CAG GGA TGA TGT TCT GG-3'.
Statistical analysis
Statistical analysis was carried out using SPSS 18.0 (SPSS, Inc., Chicago, IL, USA). All experiments were performed at least three times. The data are shown as the mean ± SD of triplicate experiments. Differences were considered statistically significant when the p value was < 0.05.
Results and Discussion
Quantification of acteoside
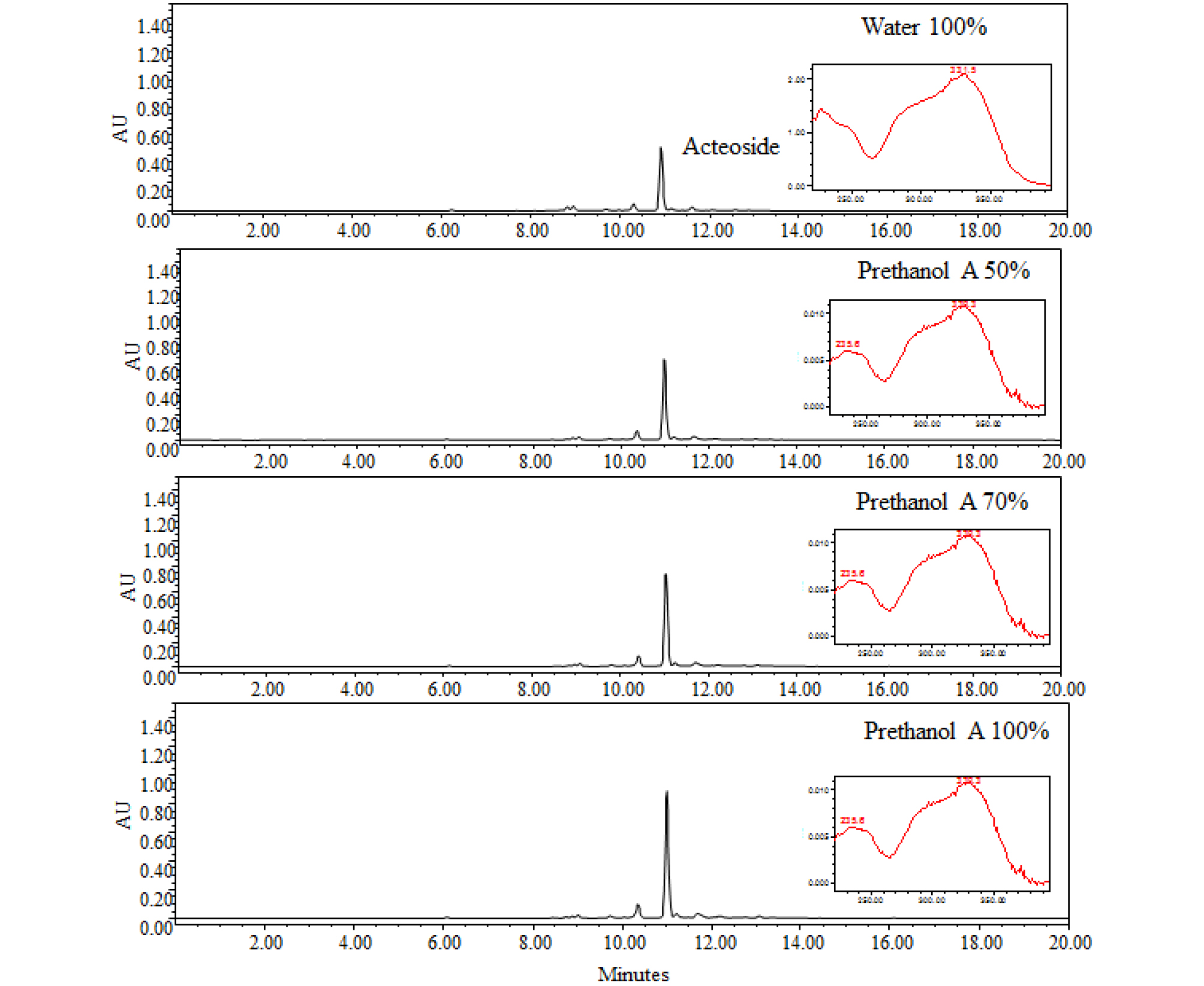
According to Bremer et al. (2002), many plants in the Oleaceae family contain acteoside. A. distichum contains various glycosides such as acteoside, isoacteoside, rutin, and hirsutrin (Oh et al., 2003), and as such its potential bioactivities have remarkable value in the industry and research fields. In our previous study, we investigated the relationship between various biological activities of A. distichum and its acteoside contents (Jang and Park, 2018). Acteoside is known to have high antioxidant (He et al., 2000), anti-inflammatory (Schlesier et al., 2002), and whitening activity (Son et al., 2011); it was identified in all extracts by HPLC-PDA method. The retention time and absorbance pattern analysis were similar to that of standard acteoside. Acteoside showed a peak at 10.6 minutes with high absorbance at 330 ㎚ (Fig. 1). The concentration of acteoside in A. distichum was 171.3 ㎎/g (water extract), 240.1 ㎎/g (50% prethanol A extract), 269.4 ㎎/g (70% prethanol A extract), and 326.1 ㎎/g (100% prethanol A extract). These results indicate that the higher the prethanol A concentration, the higher the extraction of acteoside content.
Antioxidant activity
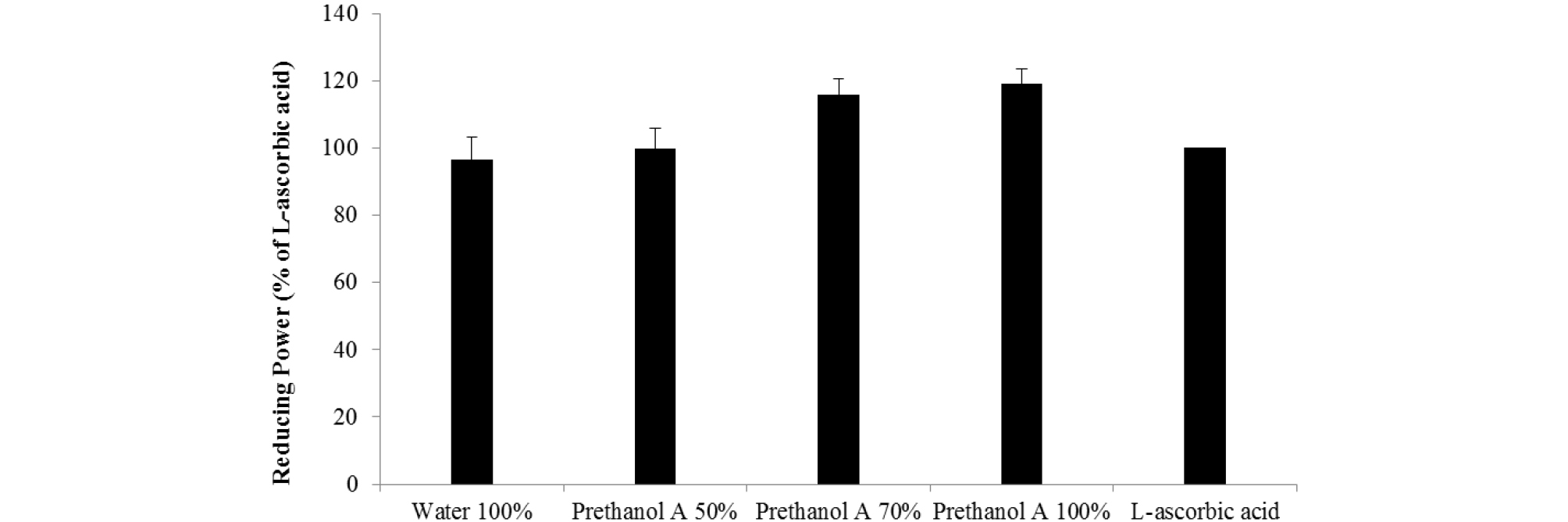
Antioxidants suppress the oxygen, electron, and hydrogen atoms generated by intracellular metabolism, protecting cells against the harmful effects of ROS, i.e. oxidative stress. If this metabolic process was impaired, oxidative damage would occur (Maxwell, 1995), potentially resulting in the development of premature skin aging, cancers, and various others conditions (Marnett, 2000). DPPH and ABTS radicals are stable chromogen compounds commonly used to measure the antioxidant activity of biological material (Que et al., 2006). As shown in Table 1, the water and prethanol A extracts of A. distichum effectively scavenged the DPPH and ABTS radicals. The IC50 values for DPPH scavenging activity were: water extract (25.1 ㎍/㎖), 50% prethanol A extract (20.4 ㎍/㎖), 70% prethanol A 70% prethanol A extract (20.2 ㎍/㎖), 100% prethanol A extract (19.0 ㎍/㎖). The IC50 values for ABTS scavenging activity were: water extract (8.05 ㎍/㎖), 50% prethanol A extract (6.69 ㎍/㎖), 70% prethanol A extract (7.45 ㎍/㎖) and 100% prethanol A extract (4.25 ㎍/㎖). Antioxidants have been attributed to the prevention of chain reactions, chelating metals, reducing capacity, radical scavenging ability, etc. educing power used a mechanism that converts yellow to green or blue by electron reduction. The reducing capacity of a compound may serve as an indicator of its potential antioxidant capacity (Ferreira et al., 2007). Reducing power was compared with L-ascorbic acid (100) relatively. As shown in Fig. 2, the reducing power of water extract (96.7), 50% prethanol A extract (99.8), 70% prethanol A extract (115.8), and 100% prethanol A extract (119.1) is shown. These results are consistent with the acteoside content of the extracts described above. Many studies have shown the antioxidant activity of acteoside (Jang and Park, 2018; Li et al., 2018) and therefore it was confirmed that acteoside content determines the antioxidant activity of extracts from A. distichum.
Table 1. DPPH and ABTS radical scavenging activity of Abeliophyllum distichum Nakai leaves according to the ratio of prethanol A in the extracts
| Prethanol A % | IC50 (Inhibitory concentration 50%, ㎍/㎖) | |
| DPPH | ABTS | |
| 0 | 25.1 | 8.05 |
| 50 | 20.4 | 6.69 |
| 70 | 20.2 | 7.45 |
| 100 | 19.0 | 4.25 |
Whitening activity
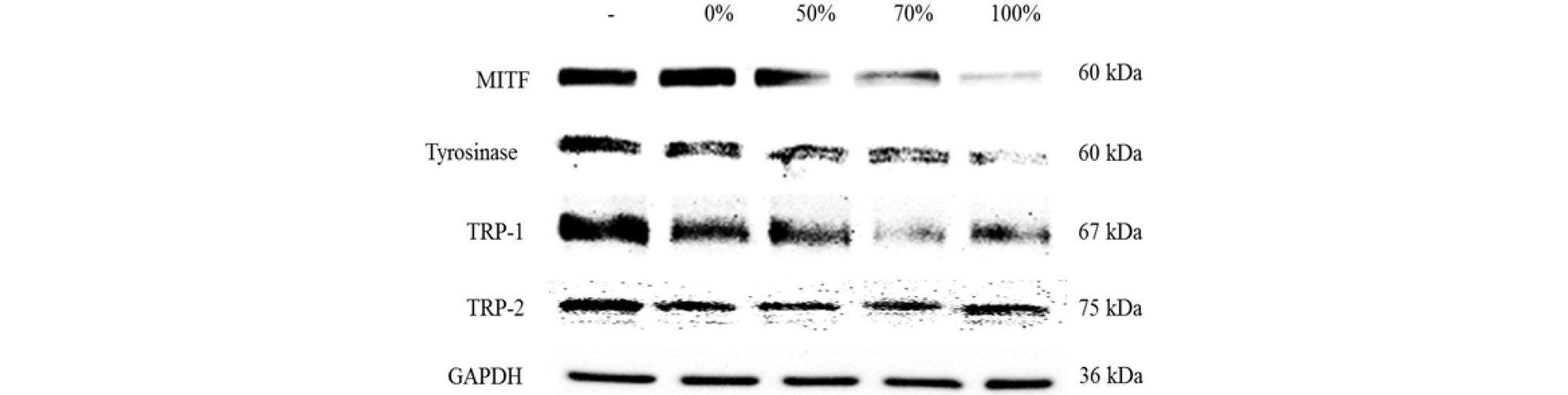
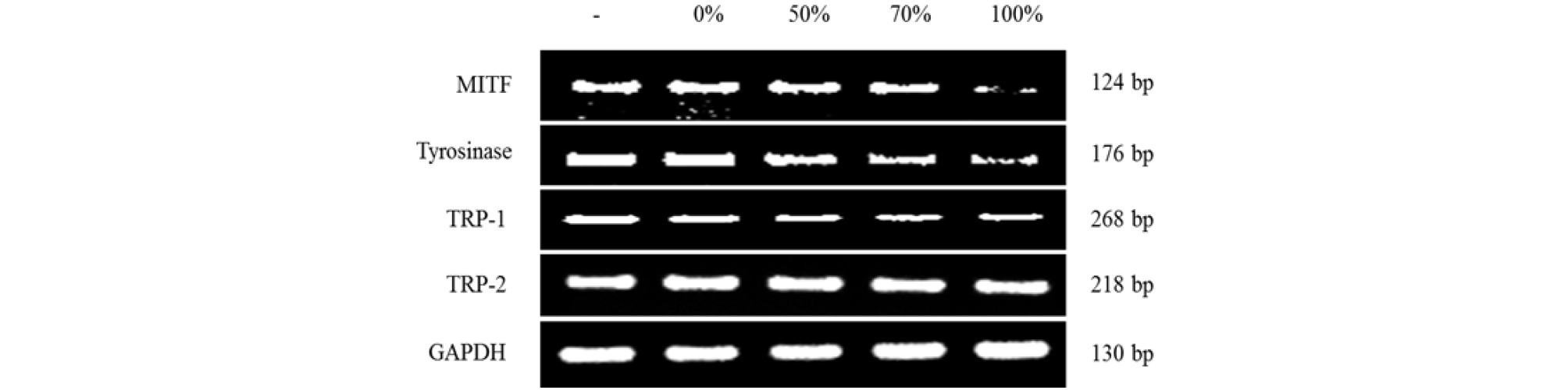
Whitening is caused by the inhibition of melanin synthesis (melanogenesis) and is a complex pathway with enzymatic catalyzed reactions. Melanogenesis is initiated with the oxidation of L-tyrosine to 3,4-dihydroxyphenylalanine (DOPA) by tyrosinase, or the biosynthesis of DOPA into DOPA-quinone by tyrosinase (Hearing and Tsukamoto, 1991; Jimenez-Cervantes et al., 1994). DOPA-quinone will serve as a substrate for the synthesis of eumelanin and pheomelanin. Inhibition of tyrosinase is important for the suppression of melanogenesis. As shown in Fig. 3, water and prethanol A extract of A. distichum inhibited the expression of tyrosinase. Inhibition of tyrosinase on prethanol A extracts were effective than water extract. The inhibition of tyrosinase on 100% prethanol A extracts was the most effective. As shown in Fig. 4, water and prethanol A extract of A. distichum inhibited the mRNA levels of tyrosinase. Prethanol A extracts were effective in inhibiting tyrosinase. TRP-1 catalyzes the oxidation of 5,6-dihydroxyindole-2-carboxylic acid (DHICA) to carboxylate indole-quinone (Hearing and Jimenez, 1987). TRP-2 known as dopachrome tautomerase (DCT) is oxidized to dihydroxyindole. These enzymes also catalyze eumelanin and pheomelanin (Que et al., 2009). As shown in Fig. 3, water and prethanol A extract of A. distichum inhibited the expression of TRP-1. Prethanol A extracts better inhibited TRP-1 than the water extracts. As shown in Fig. 4, water and prethanol A extracts of A. distichum inhibited the mRNA levels of TRP-1. Both prethanol A and water extracts inhibited TRP-1. As shown in Fig. 3 and Fig. 4, water and prethanol A extracts of A. distichum did not inhibit the expression of TRP-2. MITF, the key factor in melanogenesis, modulates tyrosinase, TRP-1, and TRP-2 (Ahn et al., 2008). Akt, p38, and ERK, which are mitogen-activated protein kinases (MAPKs), induce the transcription of MITF by binding to M-box sequences (Bentley et al., 1994). As shown in Fig. 3, water and prethanol A extracts of A. distichum inhibited the expression of MITF. As shown in Fig. 4, water and prethanol A extracts of A. distichum inhibited the mRNA levels of tyrosinase. Prethanol A inhibited tyrosinase. Inhibition of tyrosinase, TRP-1, TRP-2, and MITF could translate into a whitening effect on the skin. Therefore, antioxidants such as arbutin and ascorbic acid are used in functional cosmetics (Yang et al., 2008). Various pharmacological activities of plant flavonoid compounds are associated with antioxidant activities (Lee et al., 2005; Masaki et al., 1995). Water and prethanol A extracts of A. distichum inhibited the expression of melanogenesis-related factors. The antioxidant and whitening activity of 100% prethanol A extract was the greatest. These inhibitory effects were related to the antioxidant capacity of acteoside. In conclusion, prethanol A extract of A. distichum can be effectively utilized as a natural skin whitening agent in cosmetics and medicines.