Introduction
Materials and Methods
Reagents and Animals
Preparation of OD extracts
Scratching behavioral experiment
Induction of AD-like skin lesions and OD treatments
Measurements of skin dermatitis severity
Cytokine assay
Histamine assay
Statistical analysis
Results
Beneficial effect of OD on the scratching behaviors of mice
Beneficial effect of OD on AD symptoms of DNCB-induced AD-like skin lesions
Beneficial effect of OD on DNCB-induced IgE and histamine serum levels in mice
Beneficial effect of OD on the IL-6 and TNF-α in dorsal skin of AD mice
Discussion
Introduction
Atopic dermatitis (AD), a chronic inflammatory skin disease that occurs mainly in infancy or childhood, is characterized by intense itching, edema, erythema, thickening, severe pruritus and eczematous lesions of the skin (Buske-Kirschbaum et al., 2001). AD is caused by fine dust and various other factors such as stress, westernization of diet, and immune system imbalance. Its prevalence rate is increasing worldwide. Although its cause has not been accurately identified yet, AD is known to be a complex disorder in which several factors are involved together, including genetic factors, environmental factors, immune function imbalance, and abnormalities in skin protection (Saeki et al., 2009) AD is associated with disturbances in the skin barrier as well as immune dysregulation. Chronic AD is mostly treated with corticosteroids. However, long-term use of corticosteroids can cause serious side effects including hyperglycemia and epidermal injuries (Berke et al., 2012). Therefore, it is necessary to replace corticosteroids with natural treatments with few side effects and can be used safely. Recently, anti-atopic treatments are being developed from various natural products and medicinal plants (Jeon et al., 2021; Myung, 2020; Park et al., 2014). Salvia miltiorrhiza Bunge and coriander regulate the expression of cytokines and chemokines in the clinical state of AD and shows similar effects to dexamethasone. AD responses are processes that involve the action of multiple factors within a complex network (Sugimoto et al., 2006) AD involves various inflammatory cells including lymphocytes, eosinophils, and neutrophils with a predominant Th2 cell response. Inflammatory cytokines secreted from immune cells have been implicated in the initiation and extension of skin inflammatory reaction (Leung et al., 2004). Symptoms of AD mainly originate from response of inflammatory cells to cytokines. Such responses are pivotal factors in the pathogenesis of AD and psoriasis (Vestergaard et al., 2000). It has been reported that IL-6 and TNF-α expression are elevated in AD patients and that inhibiting these inflammatory cytokines can reduce pathological inflammation (Hussain et al., 2014)
Alternative complementary herb medicines may represent effective drugs in the treatment of various diseases. Oldenlandia diffusa (OD), a member of the Rubiaceae family, is a well-known herbal medicine used in Korean and Chinese for treating inflammatory and infectious diseases such as pneumonia and appendicitis (Ovesna et al., 2004; Wajima et al., 2016). Moreover, it has been reported that OD possesses anticancer and immunomodulatory activities (Gupta et al., 2004). OD contains many chemicals, including triterpenoids, flavonoids, ursolic acids, and oleanolic acids. Some of which have multiple effects such as anti-inflammatory, and anti-cancer activity (Kang et al., 2021; Kim et al., 2011). Although previous studies have provided evidence for the pharmacological relevance of OD, accurate information about its anti-atopic effects remain limited. Thus, this study aimed to investigate effects of OD on scratching behaviors and 2, 4-dinitrochlorobenzene (DNCB)-enhanced AD symptom in mice. Effects of OD on expression of IL-6 and TNF-α in AD-like skin lesion were also evaluated.
Materials and Methods
Reagents and Animals
DNCB, histamine, avidin peroxidase (AP), dimethyl sulfoxide (DMSO), compound 48/80, and other reagents were obtained from Sigma-Aldrich. (MO, USA). ELISA assay kit for mouse IL-6/ TNF-α/IgE were procured from BD Biosciences (CA, USA). Male ICR mice (6 weeks) were obtained from Hyochang Science (Daegu, Korea). Animals were housed at six heads per cage and allowed spontaneous intake of food and water. Animals were kept under a 12-h light/dark cycle at room temperature (24 ± 2℃) and humidity of 56 ± 10%.
Preparation of OD extracts
OD was procured from Daehak Oriental Drugstore (Iksan, Korea). Dried OD (100 g) was pulverized into a powder, decocted in 1 L of distilled water three times, and then concentrated and dried under vacuum rotary evaporator. The extract (yield, 6.84%) was filtered, freeze dried, and stored at 4˚C. The extract were dissolved in PBS and filtered through a 0.22 ㎛ syringe filter (Fisher scientific, USA).
Scratching behavioral experiment
Before the experiment, the ICR mice (n=6/each group) were put into cages for up to 30 min to allow for acclimation. Behavioral assay was performed using the method described by Sugimoto et al. (2006). We clipped the rostral area of the skin on the back of each mice. Compound 48/80 (50 ㎍/㎏) or histamine (100 ㎍/㎏) was then intradermally injected. Scratching of the injected site by the hind paw was measured and compared to scratching at other sites. Mice displayed several scratches for 1 second. A series of these scratches was counted as one incident in 30 min. In the inhibited experiments, OD (5 or 50 ㎎/㎏) was administered orally one hour before subcutaneous injection of scratching agents (histamine or compound 48/80).
Induction of AD-like skin lesions and OD treatments
DNCB was dissolved in (3:1 acetone /olive oil) and used as a sensitizer for inducing AD-like skin lesion. The dorsal skin of each mice was shaved and gauzed before sensitization. Mice were randomly divided into 4 groups (n=7/ group): a control group (vehicle), a DNCB group (negative group), OD group I (5 ㎎/㎏ OD with DNCB), and OD group II (50 ㎎/㎏ OD with DNCB). Exposed skin was treated with vehicle or 200 µL of 1% DNCB solution. On 4 day after sensitization, the dorsal skins were challenged by 200 µL of 0.5% DNCB solution three times per week. This procedure was repeated for 4 weeks and OD was orally administered every day for two weeks. After the experiment is over, mice were euthanized and their dorsal skins were separated for inflammatory cytokine analysis
Measurements of skin dermatitis severity
Dermatitis severity was evaluated by the Eczema and Severity Index scoring system: was counted by two blinded examiners using the naked eye. Scarring/dryness, edema, excoriation/erosion and erythema/hemorrhage were scored 0 (none), 1 (mild), 2 (moderate), and 3 (severe) points, respectively. Dermatitis severity scores were defined as the sum of scores for each individual.
Cytokine assay
The level of IL-6, TNF-α or IgE levels was evaluated by a modification enzyme-linked immunosorbent assay (ELISA) as described previously (Kee and Hong, 2019). Briefly, 96-well plates were coated with anti- mouse IL-6, TNF-α or IgE monoclonal Abs and incubated at 4℃ overnight. After washes, sample or a standard solution containing IL-6, TNF-α or IgE was added to each well and incubated. After washing, biotinylated anti-mouse IL-6, TNF-α or IgE Abs were reacted and incubated for 2 h. After then, we sequentially reacted with AP and 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) substrate containing H2O2. Optical density of 96-well plates was evaluated at 405 ㎚ by a microplate reader.
Histamine assay
After the experiment was completed, blood was collected from the mouse through heart blood collection, and then centrifuged at 4℃ for 10 minutes at a speed of 10,000 rpm to separate the serum. Histamine concentrations of serum were evaluated by ELISA kit (Neogen, Lexington, USA) following the manufacturer’s protocol.
Statistical analysis
Results are shown as mean ± SD and each experiment was completed at least-three times. Statistical analyses were examined using an independent t-tests and one-way ANOVA with a Tukey post hoc test. P < 0.05 was considered significant.
Results
Beneficial effect of OD on the scratching behaviors of mice
Anti-scratching effect of OD was evaluated by a histamine or compound 48/80 -induced scratching behavior animal models. When OD was orally administered 1 hour prior to compound 48/80 or histamine injections, scratching behaviors were reduced. Inhibitory rate of OD (50 ㎎/㎏) against scratching behaviors induced by compound 48/80 and histamine injection were approximately 30.81% and 27.87%, respectively (Fig. 1).

Fig. 1.
Effect of OD on scratching behavior in mice. (A and B) Extract of OD (5 or 50 ㎎/㎏) was orally administered 1 hour before intradermal injection of compound 48/80 (50 ㎍/㎏) or histamine (100 ㎍/㎏). Scratching behavior was measured as one incident of scratching for 30 minutes. Results are shown the mean ± SD of experiments. #P < 0.05; significantly different from vehicle control group, *P < 0.05; significantly different from compound 48/80 or histamine - treated group.
Beneficial effect of OD on AD symptoms of DNCB-induced AD-like skin lesions
To evaluate in vivo beneficial effects of OD in an AD model, DNCB was applied to mice. When mice received OD extract for two weeks, DNCB-enhanced AD symptoms such as dryness, edema, erosion and erythema were reduced (Fig. 2A). Additionally, we observed that skin severity scores were significantly lower in the OD treatment group than in DNCB-treated group (Fig. 2B).
Beneficial effect of OD on DNCB-induced IgE and histamine serum levels in mice
A crucial feature of AD is pathological secretion of IgE and histamine (Minami and Kamei, 2004). Total serum IgE levels are often increased in AD patients (Allam and Novak, 2006). They could be widely used as a diagnostic tool and therapeutic target. Therefore, we demonstrated the effect of OD on amount of serum IgE and histamine by ELISA kit. As shown in Fig. 3, the amount of serum IgE and histamine increased in the DNCB group. In contrast, the OD-treated group showed considerable reduction of serum IgE and histamine. Inhibitory rate of serum IgE and histamine by OD (50 ㎎/㎏) were approximately 35.4% and 22.1%, respectively (P <0.05).
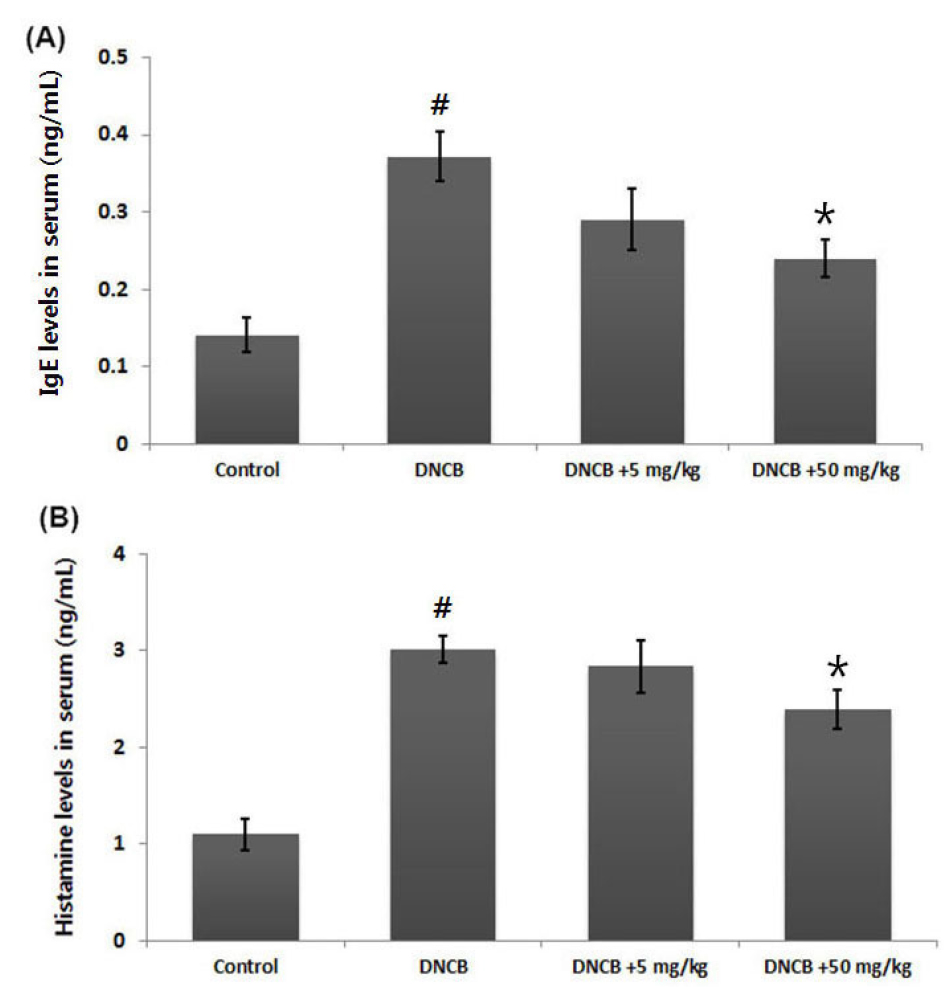
Fig. 3.
Effects of OD on DNCB-induced serum IgE and histamine levels. (A and B) Blood samples of DNCB-induced AD mice were collected. Serum of IgE and histamine amount was measured by ELISA kit according to the manufacturer’s instruction. Results are presented as mean ± SD. #P < 0.05; significantly different from vehicle control group, *P < 0.05; significantly different from DNCB- treated group.
Beneficial effect of OD on the IL-6 and TNF-α in dorsal skin of AD mice
Inhibition of IL-6 or TNF-α levels is one of the most widely accepted treatment strategies for AD (Trefzer et al., 2003). To investigate the anti-inflammatory activity of OD, we examined the regulatory effect of OD on IL-6 or TNF-α levels in the AD-like skin lesion. At the experiment is completed, skin tissues were homogenized and IL-6 and TNF-α levels was measured by ELISA. Our results show that the amount of IL-6 and TNF-α were significantly increased in dorsal skin tissues from DNCB-treated group compared to those in control group. However, administration of OD reduced DNCB-enhanced the IL-6 and TNF-α expression levels. Inhibitory rate of IL-6 and TNF-α levels by OD (50 ㎎/㎏) were approximately 34.1% and 23.8%, respectively (Fig. 4A and B).
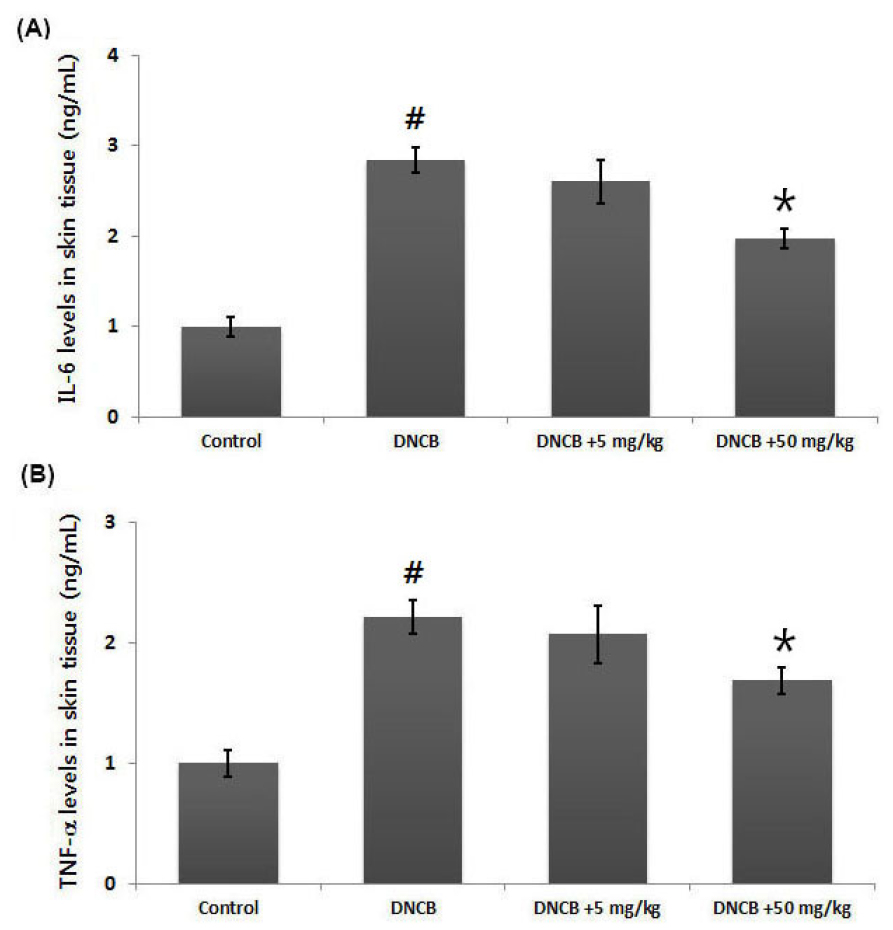
Fig. 4.
Effects of OD on IL-6 and TNF-α levels in AD-like skin lesion. (A and B) After experiment is completed, tissues were cut out and homogenized. Levels of IL-6 and TNF-α in AD-like skin lesion were measured via ELISA. Results are presented as mean ± SD. #P < 0.05; significantly different from vehicle control group, *P < 0.05; significantly different from DNCB- treated group.
Discussion
As Alternative complementary herb medicines are safe and effective, they have received increasing interest for the developing of new drugs from natural products against a wide range of diseases. Researcher has been exploring alternative treatments to replace corticosteroids for treating AD. In this study, we demonstrate that OD could attenuate histamine or compound 48/80 enhanced scratching behaviors. Additionally, OD ameliorate the DNCB-induced AD clinical symptoms and inflammatory cytokines expression in AD models.
In pathological skin conditions, histamine is involved in the induction of itching and edema (Bieber, 2008). This study focused on the manner in which OD could regulates the scratching behaviors in mice. Results showed that OD inhibited scratching induced by compound 48/80- or histamine in mice. Inhibitory rate of OD (50 ㎎/㎏) against scratching induced by compound 48/80 or histamine were approximately 30.81% and 27.87%, respectively (Fig. 1).
AD is characterized by potent skin inflammation associated with elevated levels of IgE against many types of allergens. In pathological skin conditions, histamine is involved in inducing itching and edema (Caughey, 2016). It has been reported that patients with AD have higher histamine levels than healthy subjects and that treatment with anti-histamine agents can ameliorate AD symptoms (Jemima et al., 2014). Basis on these studies, we evaluated effects of OD on IgE levels in the DNCB-induced AD model. Results showed that OD attenuates DNCB-induced histamine and IgE levels in the serum (Fig. 3). These results suggest that OD can exert an anti-atopic activity by attenuation AD clinical symptoms and serum amount of IgE and histamine.
Accumulated experimental evidence shows that inflammatory cytokines are pivotal factors in the pathogenesis of AD (Fedenko et al., 2011). It has also been reported that IL-6 and TNF-α levels are elevated in patients with AD to plays an integral role in AD pathogenesis (Ogata and Hibi, 2003). Thus, we attempted to investigate whether OD’s anti-inflammatory activity could be exerted by inhibition of these inflammatory cytokines in AD-like skin lesions. We found that DNCB-induced IL-6 and TNF-α expression was significantly reduced by administration with OD in dorsal skin tissues in AD mice. Inhibitory rate of IL-6 and TNF-α levels by OD (50 ㎎/㎏) were approximately 34.1% and 23.8%, respectively (Fig. 4). From these results, we could infer that the anti-atopic activity of OD might be associated with suppression of inflammatory cytokines.
In conclusion, OD can reduce compound 48/80 or histamine-induced scratching behaviors and DNCB-enhanced AD clinical symptoms in mice. Additionally, we demonstrated that anti-atopic effect of OD can suppress serum IgE and histamine amounts and inflammatory cytokine expression in a DNCB-induced AD model. These finding suggest experimental evidence of that OD’s potential for treating AD.





