Introduction
Materials and methods
Reagents
Preparation of the branch extracts from V. oldhamii
Cell culture and treatment
Measurement of melanin content
Measurement of cellular tyrosinase activity
SDS-PAGE and Western blot
Reverse transcriptase-polymerase chain reaction (RT-PCR)
Transfection of small interference RNA (siRNA)
Statistical analysis
Results and discussion
Effect of VOB on melanin synthesis in B16F10 cells
Effect of VOB on the expression of tyrosinase, TRP-1 and TRP-2, and tyrosinase activity in B16F10 cells
Effect of tyrosinase activity on VOB-mediated melanin biosynthesis in B16F10 cells
Introduction
The most important function of melanin in human body is to protect human skin from ultraviolet (UV) radiation (Eller et al., 1994; Zhou et al., 2012). Melanin present in skin, hair and eyes has been regarded as a major physiological defense system against solar irradiation (Zhou et al., 2012). Although excessive melanin production is known to cause skin pigmentation disorders such as melasma, freckles, moles and lentigo (Han et al., 2015), the most common hypopigmentant skin disease has been known to be vitiligo characterized by the destruction of melanocytes (Fistarol and Itin, 2010).
Derived from dopaquinone, melanin has been reported to be synthesized by more than 100 distinct genes in the melanosomes of melanocytes (Hearing and Tsukamoto, 1991; Yamaguchi et al., 2007; Schiaffino, 2010). In addition, melanin synthesis has been known to be regulated by enzymatic action such as tyrosinase, tyrosinase-related protein 1 (TRP-1) and TRP-2 (Kameyama et al., 1995, Fang et al., 2001, Park et al., 2004; Ye et al., 2010). Thus, activation of melanin synthesis through regulation of these enzymes such tyrosinase, TRP-1 and TRP-2 has been reported to be important for the treatment of hypopigmentary skin diseases such as vitiligo (Niu and Aisa, 2017).
Over the past decade, melanin synthesis promoters have been discovered and developed (Niu and Aisa, 2017). Traditional herbs and plants have been widely used for the treatment of skin hypopigmentation diseases because of their low side effects and wealth of sources (Niu and Aisa, 2017). For example, Daphne gnidium stimulated melanin synthesis through activating tyrosinase activity (Chaabane et al., 2013; Chaabane et al., 2014). Vernonia anthelmintica increased melanin contents through upregulation of tyrosinase, TRP-1 and TRP-2 in B16 cells (Tuerxuntayi et al., 2014). Besides, plants such as Cassia alata, Moricandia arvensis, Pyrostegia venusta, Ecliptae herba, Polygoni multiflori radix praeparata and Rehmanniae radix praeparata that promote melanin synthesis have been reported (Babitha et al., 2010; Skandrani et al., 2010; Moreira et al., 2012; Xu et al., 2017).
Vaccinium oldhamii Miquel (V. oldhamii) native to Korea is a deciduous shrub belonging to Ericaceae family, and grows in the southern part and Jeju island of Korea (Park et al., 2018). V. oldhamii has traditionally used to treat gonorrhea, vomiting, diarrhea, and eruption (Lee et al., 2004), and recently pharmacological activities such as anti-oxidant activity, anti-cancer activity, anti-obesity activity and anti-alzheimer activity have been reported (Lee et al., 2004; Oh and Koh, 2009; Hirotoshi et al., 2013; Park et al., 2018). Although the pharmacological properties of V. oldhamii have been reported, it is still insufficient. In this study, we firstly reported that branch extracts of V. oldhamii stimulates melanin synthesis through increasing tyrosinase activity.
Materials and methods
Reagents
Dulbecco’s Modified Eagle medium (DMEM)/F-12 1:1 Modified medium (DMEM/F-12) was purchased from Lonza (Walkersville, MD, USA). Antibodies against tyrosinase, TRP-1 and TRP-2 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies against p-ERK1/2, total-ERK1/2, p-p38, total-p38 and β-actin were purchased from Cell Signaling (Beverly, MA, USA). PD98059 (ERK1/2 inhibitor) and SB203580 (p38 inhibitor) were purchased from Calbiochem (San Diego, CA, USA). Plumbagin (tyrosinase inhibitor) and α-melanocyte-stimulating hormone (α-MSH) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Control- and tyrosinase siRNA were purchased from Santa Cruz Biotechnology. All chemicals were purchased from Fisher Scientific, unless otherwise specified.
Preparation of the branch extracts from V. oldhamii
The branches of V. oldhamii Miquel (voucher number: Jeong 201802 (ANH)) was generously provided and formally identified by Ho-Jun Son a researcher of Forest Medicinal Resources Research Center, National Institute of Forest Science, Yongju, Korea. Five grams of grounding powder from the branches of V. oldhamii were extracted with 100 ㎖ of 70% ethanol for 72 h under stirring at room temperature. After 72 h, the ethanol-soluble fraction was filtered and concentrated to approximately 30 ㎖ volume using a vacuum evaporator and then freeze-dried. The ethanol extracts from the branches of V. oldhamii (VOB) were kept in a refrigerator (-80℃) until use.
Cell culture and treatment
Murine melanoma cell line, B16F10 was purchased from ATCC (Manassas, VA, USA). B16F10 cells were maintained at 37℃ under a humidified atmosphere of 5% CO2 using DMEM/F- 12 media containing 10% fetal bovine serum (FBS), 100 U/㎖ penicillin and 100 ㎍/㎖ streptomycin. VOB was dissolved in dimethyl sulfoxide (DMSO) and treated to cells. DMSO was used as a control and the final DMSO concentration did not exceed 0.1% (v/v).
Measurement of melanin content
The effect of VOB on the melanin synthesis was evaluated by measuring intracellular and extracellular melanin content. B16F10 cells were plated in phenol red-free DMEM containing 10% FBS. B16F10 cells were treated with VOB at the indicated concentrations in absence or presence of α-MSH for 72 h. After 72 h, the absorbance of the cell culture media for the extracellular melanin was measured at a wavelength of 475 ㎚ using UV/Visible spectrophotometer (Human Cop., Xma-3000PC, Seoul, Korea). For the intracellular melanin, the cultured cells were dissolved in 1 N NaOH containing 10% DMSO at 80℃ for 1 h, and then absorbance was measured at a wavelength of 475 ㎚ using UV/Visible spectrophotometer (Human Cop., Xma- 3000PC, Seoul, Korea).
Measurement of cellular tyrosinase activity
B16F10 cells were treated with VOB at the indicated concentrations in absence or presence of α-MSH for 72 h. After 72 h, B16F10 cells were washed with 1 × PBS three times and then lysed with 0.5% Triton X-100. Protein concentration was determined by the bicinchoninic acid (BCA) protein assay (Pierce, Rockford, IL, USA). Thirty microgram of protein was incubated with 2 mM L-DOPA and 0.1 M 1 × PBS (pH 6.8) for 1 h at 37℃. After 1 h, absorbance was measured at a wavelength of 475 ㎚ using UV/Visible spectrophotometer (Human Cop., Xma-3000PC, Seoul, Korea).
SDS-PAGE and Western blot
After each treatment, B16F10 cells were washed with 1× phosphate-buffered saline (PBS), and lysed in radioimmunoprecipitation assay (RIPA) buffer (Boston Bio Products, Ashland, MA, USA) supplemented with protease inhibitor cocktail (Sigma- Aldrich) and phosphatase inhibitor cocktail (Sigma-Aldrich), and centrifuged at 15,000 × rpm for 10 min at 4℃. Protein concentration was determined by the bicinchoninic acid (BCA) protein assay (Pierce, Rockford, IL, USA). The proteins were separated on SDS-PAGE and transferred to PVDF membrane (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The membranes were blocked for non-specific binding with 5% non-fat dry milk in Tris-buffered saline containing 0.05% Tween 20 (TBS-T) for 1h at room temperature and then incubated with specific primary antibodies in 5% non-fat dry milk at 4℃ overnight. After three washes with TBS-T, the blots were incubated with horse radish peroxidase (HRP)-conjugated immunoglobulin G (IgG) for 1 h at room temperature and chemiluminescence was detected with ECL Western blotting substrate (Amersham Biosciences, Piscataway, NJ, USA) and visualized using LI-COR C-DiGit Blot Scanner (Li-COR Biosciences, Lincoln, NE, USA).
Reverse transcriptase-polymerase chain reaction (RT-PCR)
After each treatment, total RNA was prepared using a RNeasy Mini Kit (Qiagen, Valencia, CA, USA) and total RNA (1 µg) was reverse-transcribed using a Verso cDNA Kit (Thermo Scientific, Pittsburgh, PA, USA) according to the manufacturer's protocol for cDNA synthesis. PCR was carried out using PCR Master Mix Kit (Promega, Madison, WI, USA) with human primers for tyrosinase, TRP-1, TRP-2 and GAPDH as followed : tyrosinase: forward 5'-GGCCAGCTTTCAGGCAGAGGT-3' and reverse 5'-TGGTGCTTCATGGGCAAAATC-3', TRP-1: forward 5'-AAGCAGACATCCAACAACACTAG-3' and reverse 5'-GCAAGAGTTCAGAACACAGGTC-3', TRP-2: forward 5'-GCAAGAGATACACGGAGGAAG-3' and reverse 5'-CTAAGGCATCATCATCATCACTAC-3', GAPDH: forward 5'-GGCA TGGCCTTCCGTGT-3' and reverse 5'-GGTTTCTCCAGGCGGCA-3'. The following PCR reaction conditions were used: 1 cycle of (3 min at 94℃ for denaturation), 30 cycles of (30 s at 94℃ for denaturation, 30 s at 60℃ for annealing, and 30 s at 72℃ for elongation), and 1 cycle of (5 min for extension at 72℃).
Transfection of small interference RNA (siRNA)
B16F10 cells were plated in 6-well plates and incubated overnight. B16F10 cells were transfected with control siRNA and tyrosinase siRNA for 48 h at a concentration of 100 nM using TransIT-TKO transfection reagent (Mirus, Madison, WI) according to the manufacturer's instruction.
Statistical analysis
All the data are shown as mean ± SD (standard deviation). Statistical significance was determined by Student’s t-test. Differences with *P or #P < 0.05 was considered statistically significant.
Results and discussion
Effect of VOB on melanin synthesis in B16F10 cells
To evaluate whether VOB stimulates or inhibits melanin synthesis, we measured extracellular and intracellular melanin contents at 72 h after the treatment of VOB to B16F10 cells in presence of α-MSH (100 nM). As shown in Fig. 1A, the treatment of α-MSH alone increased extracellular and intracellular melanin contents compared to the cells without the treatment of α-MSH and VOB. However, VOB treatment in presence of α- MSH more increased extracellular and intracellular melanin contents compared to the cells treated with α-MSH alone. In addition, we observed that VOB treatment in absence of α- MSH elevated extracellular and intracellular melanin contents compared to the cells without VOB treatment (Fig. 1B). These data indicate that VOB may promote melanin synthesis.
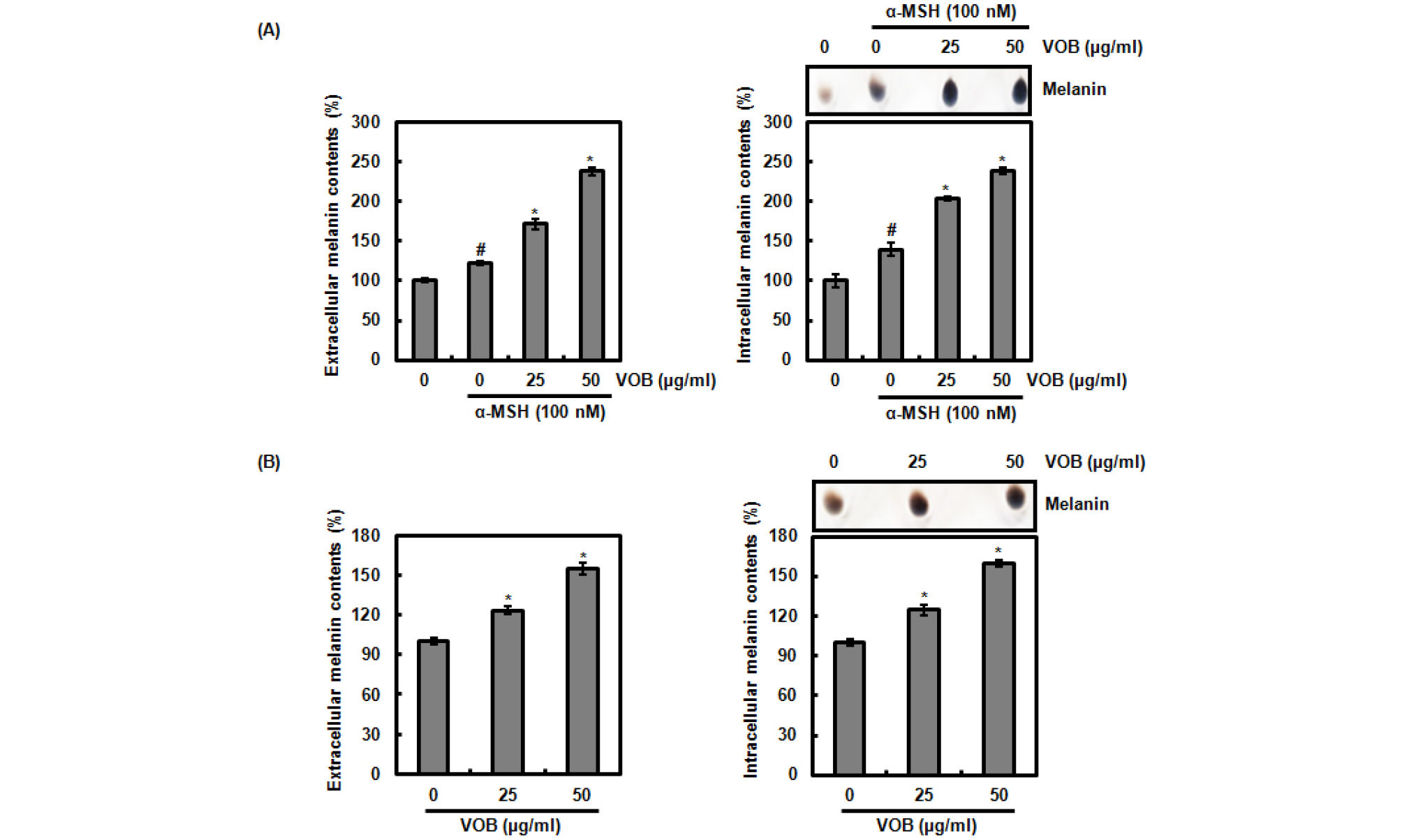
Fig. 1.
Effect of VOB on melanin synthesis in B16F10 cells. (A) B16F10 cells were pretreated with VOB for 2 h and then co-treated with α-MSH (100 nM) for 72 h. (B) B16F10 cells were treated with VOB for 72 h. Then, extracellular and intracellular melanin contents were measured at 475 nm. #P < 0.05 compared to cells without α-MSH and VOB, and *P < 0.05 compared to cells without α-MSH.
Effect of VOB on the expression of tyrosinase, TRP-1 and TRP-2, and tyrosinase activity in B16F10 cells
Melanin biosynthesis begins with the conversion of tyrosine to 3,4-dihydroxyphenylalanine (DOPA) by tyrosinase and subsequent oxidation to DOPAquinone catalyzed by tyrosinase (Hearing and Jimenez, 1987). Then, DOPA quinone is converted to DOPAchrome by auto-oxidation. DOPAchrome is converted to 5,6-dihydroxyindole-2-carboxylic acid (DHICA) by TRP-2 (Yokoyama et al., 1994) and subsequently DHICA is oxidized to a carboxylated indole-quinone by TRP-2 (Kobayashi et al., 1994) to form brown-black pigment (eumelanin). Thus, tyrosinase, TRP-1 and TRP-2 have long been known to be critical regulators of melanin biosynthesis (del Marmol and Beermann, 1996). To investigate whether VOB increases the expression of tyrosinase, TRP-1 and TRP-2, B16F10 cells were treated with VOB for 72 h in absence of α-MSH, and then RT-PCR and western blot analysis were performed. As shown in Fig. 2A. VOB did not affect both mRNA and protein level of tyrosinase and TRP-1. However, TRP-2 protein level was decreased by VOB treatment but not mRNA level. Although the expression of tyrosinase was not changed by VOB, we evaluated whether VOB affects tyrosinase activity because tyrosinase activity is very important for melanin biosynthesis (Niu and Aisa, 2017). As shown in Fig. 2B, VOB was observed to activate cellular tyrosinase activity. From these results, it is thought that VOB may stimulate melanin biosynthesis through tyrosinase activation. In addition, VOB-mediated downregulation of TRP-2 regulating the conversion of DOPAchrome to DHICA may induce the conversion of DOPAchrome to dihydroxyindole (DHI), which in turn contributes to conversion of DHI to eumelanin by tyrosinase.
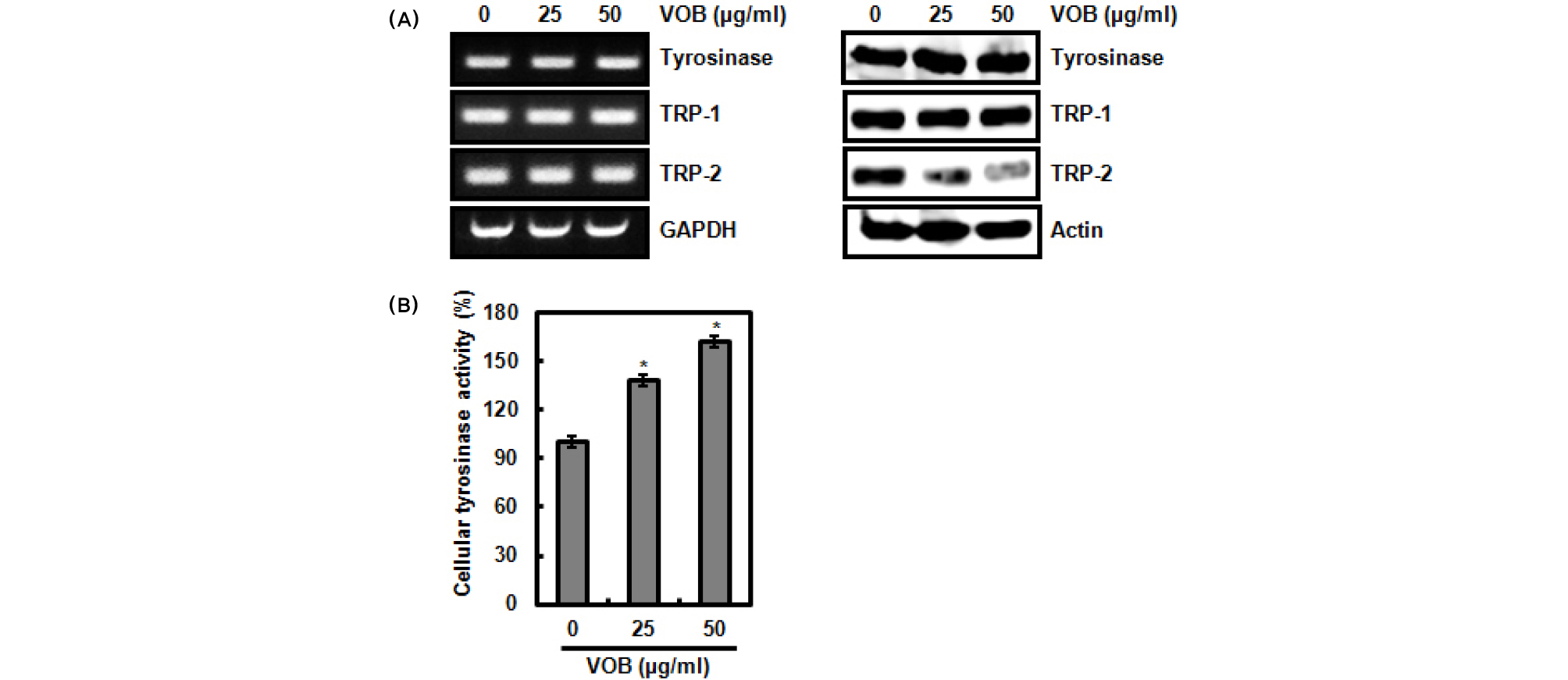
Fig. 2.
Effect of VOB on the expression of tyrosinase, TRP-1 and TRP-2, and tyrosinase activity. (A and B) B16F10 cells were treated with VOB for 72 h in absence of α-MSH. For RT-PCR analysis, Total RNA was isolated and RT-PCR was performed against tyrosinase, TRP-1, TRP-2 and GAPDH. For Western blot analysis, Cell lysates were subjected to SDS-PAGE and Western blot was performed using antibodies against tyrosinase, TRP-1, TRP-2 and actin. (C) B16F10 cells were treated with VOB for 72 h in absence of α-MSH. After 72 h, tyrosinase activity was measured as described in materials and methods. *P < 0.05 compared to cells without VOB.
Effect of tyrosinase activity on VOB-mediated melanin biosynthesis in B16F10 cells
To evaluate whether VOB-mediated melanin biosynthesis is dependent on tyrosinase activity. B16F10 cells were pretreated with plumbagin for 2 h and then co-treated with VOB for 72 h. Plumbagin as a plant-derived secondary metabolite has been reported to inhibit melanin biosynthesis through inhibiting tyrosinase activity but not expression of tyrosinase, TRP-1 and TRP-2 (Oh et al., 2017). Thus, Plumbagin was selected for the inhibition of tyrosinase activity. As shown in Fig. 3A, the treatment of VOB alone increased the intracellular melanin content and tyrosinase activity. However, the presence of plumbagin blocked VOB- mediated increase of melanin content and tyrosinase activity. In addition, knockdown of tyrosinase by tyrosinase siRNA attenuated VOB-mediated increase of melanin content and tyrosinase activity. These data indicate that VOB-mediated melanin biosynthesis may be dependent on tyrosinase activity.
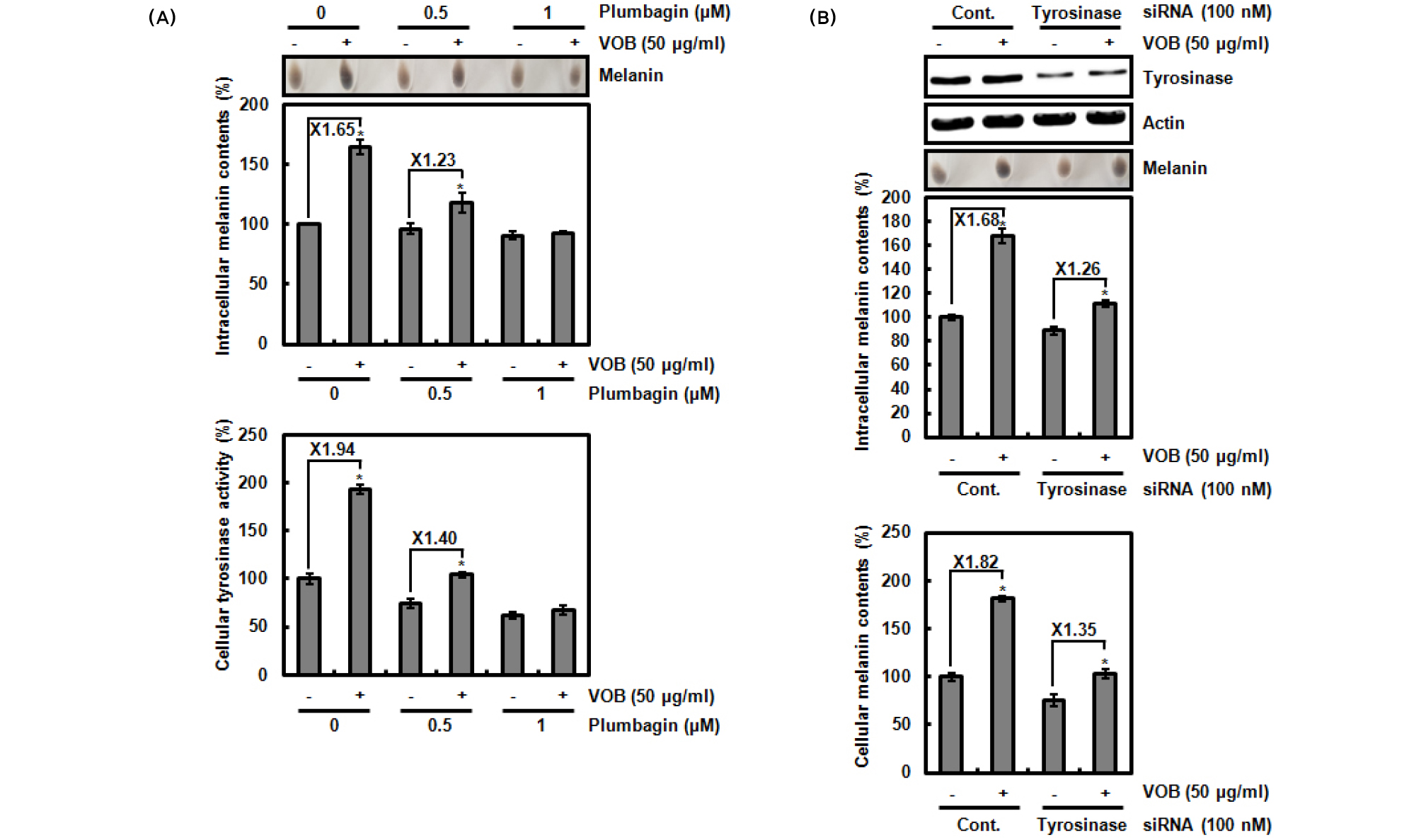
Fig. 3.
Effect of tyrosinase activity on VOB-mediated melanin biosynthesis in B16F10 cells. (A) B16F10 cells were treated with plumbagin for 2 h and then co-treated with VOB for 72 h. After 72 h, intracellular melanin content and tyrosinase activity were measured as described in materials and methods. *P < 0.05 compared to cells without VOB. (B) B16F10 cells were transfected with control- and tyrosinase siRNA for 48 h and then co-treated with VOB for 72 h. After 72 h, Cell lysates were subjected to SDS-PAGE and Western blot was performed using antibody against tyrosinase and actin. Intracellular melanin content and tyrosinase activity were measured as described in materials and methods. *P < 0.05 compared to cells without VOB.




