Introduction
Materials and Methods
Materials
Animal experiments
Preparation of hepatic ischemia-reperfusion model
Specimen collection and testing
Measurement of biochemical parameters
Immunohistochemistry
Statistical analysis
Results
Effects of GLEVC injection on ALT and AST level in serum
Effects of GLEVC on activities of serum SOD, MDA in HIRI rat
Effects of GLEVC on SOD activity and MDA content in tissue of HIRI rats
Effects of GLEVC on expression of Bax and Bcl-2 in liver tissue of HIRI rats
Discussion
Introduction
Clamping of the portal pedicle along with the inferior vena cava below was useful methods on the clinical and/or experimental liver-related disease model. These tools were conventional protocols for resection of tumorous liver by close the hepatic veins or the vena cava (Serracino-Inglott et al., 2001; Huguet et al., 1992; Garden, 1994). A period of ischemia is required for a many surgical procedures on the liver such as transplantation, dealing with extensive hepatic trauma, and resecting large intrahepatic lesions (Huguet et al., 1992; Delva et al., 1989). Restoration of blood after liver ischemia, the liver subjected to a further insult, accelerating the cellular injury by ischemia. This is termed ischemia-reperfusion (IR) injury, and in the field of hepatic transplantation, it covers a various problems with the common clinical phenomenon of a poorly functioning graft (Henderson, 1999; Serracino-Inglott et al., 2001).
Ginko biloba, has various advanced properties as antioxidant as well as clinical benefits in several conditions such as ischemia, epilepsy, and peripheral nerve damage (Brondino et al., 2013). These resources has been used in almost country to treat circulatory disorders, asthma, tinnitus, vertigo, and cognitive problems (Kleijnen and Knipschild, 1992). In extract of leaves of Ginko biloba, terpenoids, flavonol glycosides and proanthocyanidins were main effective molecules and its properties were proposed by in vitro and in vivo experiments (Brondino et al., 2013). Indeed, injection of similar extracts in previous reports shows that low dosages given only partial ameliorative effects on HIRI-induced hapatic injury (Huang, 2011). Therefore, these protocols were requested novel trial like combinative therapies with other effective molecules such as vitamin C for application to medical purposes.
Vitamin C, identified as L-ascorbic acid, is a found in various fresh plant resources and used as an essential dietary supplement, and then required for the functioning of several enzymes and/or many functional system of body as antioxidants (Mandl et al., 2009). In other reports, described the effect of ascorbic acid (AA) as alterations resources in hepatic secretory and microsomal functions during hepatic ischemia and reperfusion (Seo and Lee, 2002).
In this study, we investigated the advanced protective effects of both GLEVC in HIRI model. Specifically, we examined the abilities of each to regulate hepatic-related enzymes activities in blood and inflammatory molecules expression, using a rat model of ischemia/reperfusion injury induced by vascular clamping.
Materials and Methods
Materials
The Ginko biloba leaves extract was obtained from Zhongho International Co Ltd (Cat. # HC20140019, Taiwan). Ascorbic acid (vitamin C) and other reagents were obtained from Sigma-Aldrich (MO, USA). Both specimens were aliquots and stored in deep freezer until use. To injection, GLE (as 17.5 ㎎/ml stock solution) was mixed with vitamin C-saline (as ×10 stock) or same volume of saline before use. The final dosage of GLE and vitamin C were 31.5 ㎎/㎏ (Huang, 2011) and 300 ㎎/㎏ (Mandl et al., 2009), respectively.
Animal experiments
The 40 male Wistar rats (270-300 g) were purchased from Charles River (Shanghai, China). The experiments were performed in accordance with the principles and with the approval of the Ethics Committee of the YanBian University, China (Approval No. Z23021162). All animals maintained in a temperature/humidity-controlled room with a light/dark (12 h/12 h) cycle, and acclimatized to the laboratory environment while housed in individual cages for 1 week before the experiments. Animals were randomly divided into four groups: sham operation group (normal group): Only open without occlusion of blood vessels; ischemia-reperfusion group (control group); treatment group (experimental group). Sham group and ischemia-reperfusion group calculated according to the weight given saline.
Preparation of hepatic ischemia-reperfusion model
Male Wistar rats weighing 270-300 g of fasting before surgery 12 h, After weighing accuracy with 10% chloral hydrate 4 ㎖/㎏ intraperitoneal injection to anesthetized rats. After induction of anesthesia with intramuscular ketamine hydrochloride (100 ㎎/㎏), The GLEVC injected slow intravenously with indicated dose (2 ㎖ per rat) to experimental groups during 15 min. Normal and control group were also injected equal volume of saline by same procedures. After 30 minute of injection with GLEVC or saline, an indwelling intravenous line was placed via the right external jugular vein for serial blood sampling and administration of intravenous fluids and medications. By positioning the catheter tip in the suprahepatic vena cava just above the dome of the liver, immediately posthepatic blood samples were obtained; correct catheter position was confirmed at laparotomy. After intravenous heparinization with 100 U/100 g of body weight, midline laparotomy was performed. Hepatic ischemia was initiated by application of a Heifitz clip, ischemia was maintained for 60 min, and the Heifitz clip was then removed at a second laparotomy. Intravenous lactated Ringer's solution in a dose of 0.75 ㎖ was administered to replace operative fluid and blood losses. Sham-operated control animals were treated in an identical fashion with the omission of vascular occlusion (Colletti et al., 1990).
Specimen collection and testing
Animals after reperfusion 2h, inferior vena cava blood 2 ㎖ and centrifuged at 3,000 rpm for 10 min for separation of serum. Detectable levels ALT, AST, SOD activity and MDA were measured. The left lobe of liver tissue were obtained and moved to 0.85% ice-cold saline. The serially diluted liver homogenate were used for detecting protein content, SOD activity, MDA, GSH content. Another portion of liver lobe were fixed with 10% neutral formalin for 48h at 4°C, embedded in paraffin for immunohistochemistry and measured Bcl-2 and Bax protein.
Measurement of biochemical parameters
To assess hepatic function and injury after liver ischemia, we measured serum ALT and AST levels were measured using a clinical chemistry system (Bayer Co. Tarrytown, NY). SOD activity was measured through the inhibition of nitroblue tetrazolium (NBT) reduction by O2.- generated by the xanthine/xanthine oxidase system. One SOD activity unit was defined as the enzyme amount causing 50% inhibition in 1 ㎖ reaction solution per milligram tissue protein and the result expressed as Units/㎎ protein. MDA levels of serum and liver tissues were assayed by the measurement of thiobarbituric acid-reactive substances (TBARS) levels at 532 ㎚. Results were expressed as μmol/L serum and nmol/㎎ protein, respectively.
Immunohistochemistry
For immunohistochemistry, skin tissues were fixed in 10% formaldehyde, embedded in paraffin and sectioned at 4 ㎛ thickness. The sections were deparaffinized and antigen-retrieval was performed in sodium-citrate buffer (10 mM, pH 6.0) for 10 minutes at 90°C. Sections were blocked in 1% BSA in PBS for 30 minute and incubated 2 hours with a polyclonal mouse antibody against indicated antibodies. After 4 times washes for 5 minutes each with PBS, sections were incubated with horseradish peroxidase-conjugated secondary antibodies. Stained sections were mounted and visualized with a Leica microscope with digital camera (DC200, Germany) (Park et al., 2015).
Statistical analysis
All data were expressed as mean ± SD, and differences between groups were analyzed using student t-test. All analyses were performed using SPSS 10.0 (SPSS Inc. USA). Each value was the mean of at least 3 separate experiments in each group, and data with different superscript letters are significantly different when p value is less than 0.05.
Results
Effects of GLEVC injection on ALT and AST level in serum
To investigate the therapeutic potential of GLEVC on HIRI-induced liver damages, we first measured only showing serum ALT and AST levels compared with normal and HIRI processed rats. GLEVC has been shown to reduce serum ALT and AST levels increases on HIRI rats (Table 1). HIRI rat shown increase of ALT and AST levels in serum however GLEVC reduced levels significantly compared than GLE group. Thus, these results suggest that GLEVC was more effective than GLE treatment on HIRI mediated liver cells damages.
Effects of GLEVC on activities of serum SOD, MDA in HIRI rat
To examine the effects of GLEVC on HIRI-mediated liver damage, we measured serum SOD and MDA levels. In Table 2, significant reduces of SOD levels were detected in HIRI control group compared with the normal group. However, those were markedly reduced by GLEVC compared than GLE group. Whereas the MDA levels was significantly increased by HIRI processes. In contrast, GLEVC administration were reduced serum MDA levels compared with HIRI processed rats in a dose dependent manner. Although GLEVC shown significant results but GLE also shown recovering effects against the HIRI-mediated increase in MDA level. These results suggest that improving effects of GLEVC donated as more potent antioxidant against oxidative stress by HIRI-induced liver damages.
Table 2. The ameliorative effect of GLE and GLEVEC on SOD activity and MDA content in serum (Mean ± SD) |
|
Effects of GLEVC on SOD activity and MDA content in tissue of HIRI rats
Similarly, serum SOD activity, SOD activity in HIRI-processed liver tissue was also reduced. In Table 3, SOD activity in liver tissue of HIRI group was reduced than normal group but these activities were significantly recovered by GLEVC process and GLE administration. Indeed, HIRI-mediated increases of MDA contents in liver tissue were significantly reduced in GLEVC and even shows significant changes GLE treated group also. Thus, these results suggest that GLEVC was effective to HIRI-mediated liver damage via anti-oxidative mechanisms and parallel cellular signaling.
Table 3. The ameliorative effect of GLE and GLEVEC on SOD activity, MDA content and GSH level in tissues (Mean ± SD) |
|
Effects of GLEVC on expression of Bax and Bcl-2 in liver tissue of HIRI rats
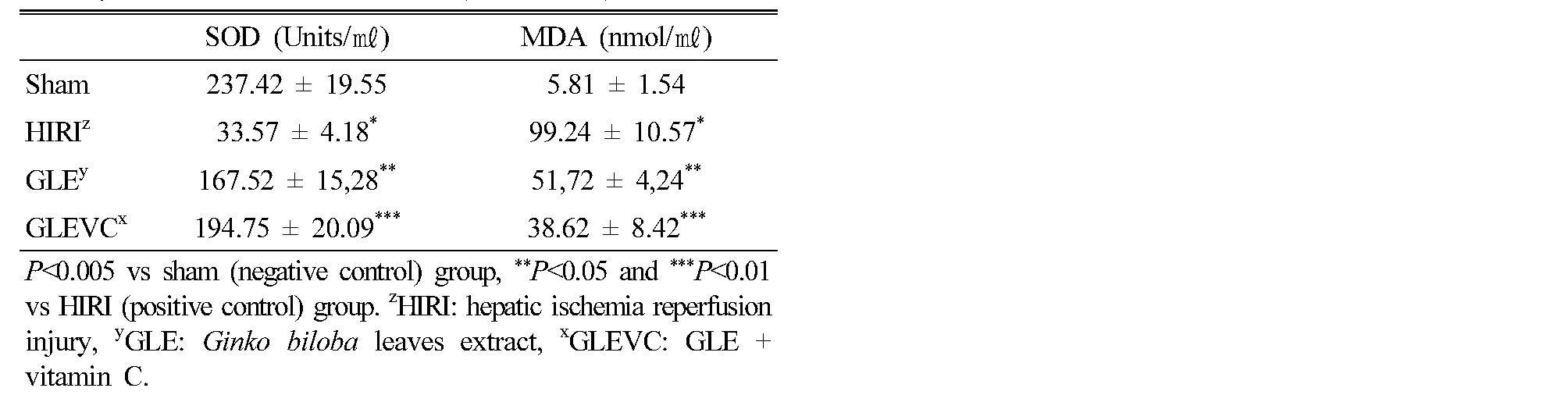
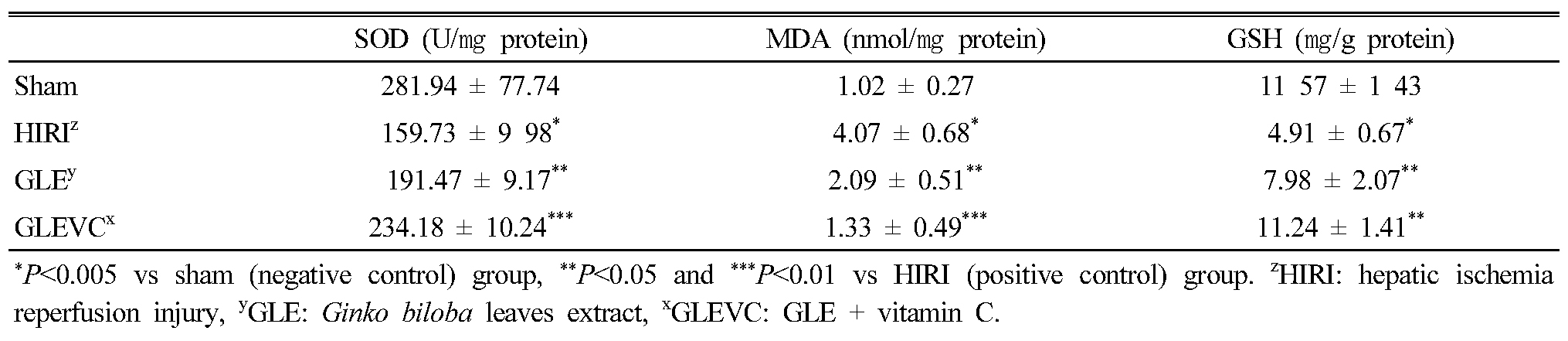
The experimental results show Bax expression (Fig. 1) and Bcl-2 (Fig. 2). In HIRI group, Bax protein expression was significantly increased in liver tissue compared with normal group. GLE administration could slightly reduce the expression of Bax protein, compared with HIRI group was statistically significant but GLEVC treatment was markedly reduced Bax expression in liver tissue (Fig. 1). Whereas, HIRI processes were reduces Bcl-2 expression, compared with normal group. These increases of Bcl-2 were partially reversed by GLE administration, but GLEVC treatment shown potent effects on Bcl-2 rescue in liver tissue.
Discussion
The present study, we observed the protective effects of GLEVC in HIRI model. The Ginko biloba leaves was widely adopted as a traditional medicine in Asia, Europe, western countries and investigated by alternative medicine in worldwide (Yoshikawa et al., 1999). GLE contained various biological effective molecules such as different flavone glycosides and terpenoids. GLE has an antioxidant action, anti-inflammatory effects, improving effects on blood flow (Kleijnen and Knipschild, 1992). However, high dose of GLE has a better effects in a dose dependent manner (Huang, 2011) but should be concern risks for medical application cause of unidentifiable side effects, therefore required relative low dose and could find alternative tools in same model. In these study, we chosen relative low dose of GLE (Huang, 2011) and combined vitamin C as a typical antioxidant. Indeed, both components can purchased on the market and available to modifies as edible foods such as tea, capsule, or powder but these results suggested that intravenous injects also available not like western countries.
Vitamin C (ascorbic acid) is reported strong antioxidant and protect tissue from dangerous oxidational products and maintained important enzymes in their reduced states. Moreover, vitamin C can scavenge singlet oxygens and reaction to harmful products, scavenging superoxide and hydroxyl radicals (Padh, 1990; Zeng et al., 1991). Therefore combination therapy of vitamic C was available methods in various disease managements. In other report, vitamins were used during open heart surgery for protection of ischemia-reperfusion damages (Barta et al., 1991).
Pathophysiologically, GLEVC rescued anti-apoptotic molecule (Bcl-2) expression and reduced the apoptotic signaling molecule (Bax) in liver tissue on HIRI model mice (Fig. 1 and 2), Although, we measured major parameters on HIRI-induced hepatic damage model as a surgery medication, these study required more quantitative experiments for identification of action mechanism. Therefore, needs to other progressive methods such as real-time PCR (qPCR) and/or western blotting in various dose condition in further study. Indeed, although GLE has been shown to mediate ameliorative effects in rat livers ischemia/reperfusion (Lim et al., 2013), it remains unknown detail mechanisms can affect liver HIRI in vivo. Therefore, we should found noble pathways such as cAMP/Ca2+ sigmal like previous our report (Xie et al., 2017) with hepatic ischemia/reperfusion condition in pathophysiologic and experimental model. In conclusion, these results suggest that GLEVC exert potent ameliorative effects and inhibit the damage signaling on hepatic ischemia/reperfusion injury via antioxidation signaling cascade.