Introduction
Materials and Methods
Samples preparation
Total polyphenol determination
Total flavonoid determination
Assay of DPPH radical scavenging rate
Assay of cytotoxicity on human cancer cell lines
Assay of cytotoxicity on 3T3-L1 cell
Data analysis
Results and Discussion
Total polyphenol and flavonoid contents
DPPH radical scavenging activity
Cytotoxicity on human cancer cell
Cytotoxicity on 3T3-L1 cell
Introduction
Codonopsis lanceolata (C. lanceolata) is a perennial flowering plant belonging to the family Campanulaceae and is grown commercially in East Asia. The roots of C. lanceolata have been used as a tonic crude drug and an edible plant in Korea, and mainly contain triterpenoid saponins including codonolaside I-V, lancemaside A-G. Their saponins have shown anti-inflammatory effects such as bronchitis and cough, insomnia, and hypomnesia (Boo et al., 2016). Lancemaside A, which is a main constituent of C. lanceolata was reported to potently inhibit LPS-stimulated, TLR-4-linked NF-jB activation of 293-hTLR4-hemagglutinin cells (Joh et al., 2010). C. lanceolata is well known to affect various pharmacological effects for human health and its consumption is increasing. Recently, plant and plant-derived products are treated a part of the healthcare system by applying the bioactive phytochemicals. Previous scientific findings have demonstrated that traditional medicinal plants retain in vitro mutagenic or toxic and carcinogenic properties, thus it is crucial to examine medicinal plants for their cytotoxicity. The cytotoxicity evaluation of plants is a major subject in pharmaceutical studies, particularly in the area of cancer research (Cuyacot et al., 2014). As an herb, C. lanceolata is widely used in food preparation, but its medicinal application has not been explored yet in South Korea (Wang et al., 2011). Medicinal plant may aslo reduce the risk of oxidative stress and cell damage (Guizani et al., 2013). Antioxidant compounds in food play an important role as a health protecting factor. Scientific evidence suggests that antioxidants reduce the risk for chronic diseases, including cancer and heart disease. Most of the antioxidant compounds in a typical diet are derived from plant sources that belong to various classes of compounds with a wide variety of physical and chemical properties. The main characteristic of an antioxidant is its ability to eliminate free radicals. Highly reactive free radicals and oxygen species are present in biological systems from a wide variety of sources. This study was executed to investigate the in vitro anti- obesity activity of different extract concentation of EtOH in C. lanceolata. Confluent 3T3-L1 preadipocytes start differentiation induced by adipogenic inducers including fetal bovine serum (FBS), dexamethasone (Dex), isobutylmethylxanthine (IBMX) and insulin. This physiological process of cell differentiation converts preadipocytes to adipocytes, which is called adipogenesis. Lipid accumulation in human adipose tissues is determined by the balance between fat synthesis (lipogenesis) and breakdown (lipolysis). Lipogenesis usually occurs in the liver and adipose tissues. (Li, 2014). Weight loss and weight maintenance are the important goals of obesity treatment which can be done by several ways including the use of lipase inhibitors. Recently, lipase inhibitors from plants such as saponins, polyphenolic compounds and terpenes have garnered increasing attention since they showed sufficient activity (Rahul and Kamlesh, 2007). The aim of this study was to evaluate the total polyphenol, flavonoid contents, antioxidant activity and the cytotoxic effect in human cancer cell, 3T3-L1 cell from C. lanceolata extracts at various ethanol concentration.
Materials and Methods
Samples preparation
C. lanceolata grown in Jeju region was purchased from a farm. The roots of C. lanceolata were freeze dried and ground to a fine powder. The powder was stored at -20°C until further analyses. The freeze dried powder was immersed in 30%, 50%, 70% and 100% ethanol and the filtrate was collected thrice with constant stirring of the mixture at every 24 h interval for 72 h. The filtrate was then concentrated under reduced pressure at 45°C using a vacuum rotary evaporator (IKA® RV 10 Basic Digital, IKA Co., Germany). The concentrated extract was stored at -20°C until further analysis.
Total polyphenol determination
Total phenols were determined by the modified method the Folin- Ciocalteu assay (Singleton and Rossi, 1965). Freeze-dried samples were extracted with methanol, the extract was concentrated under reduced pressure, and freeze-dried in powder. 1 ㎎ freeze-dried powder dissolved in 95% methanol, and 500 ㎕ of Folin- Ciocalteu reagent were added to a 25 ㎖ volumetric flask and were mixed for 5 minute at 30°C in water bath. 500 ㎕ saturated solution of 7.5% Na2CO3 was added to the mixture, and then was incubated for 1 hour at room temperature, and the absorbance was read at 725 ㎚ using a spectrophotometer (Biochrom Co., England). Total phenolic of the sample was expressed as mg chlorogenic acid equivalent in 1 g dry weight of sample extract.
Total flavonoid determination
Total flavonoid was measured using the modified method that previously described (Zhishen et al., 1999). Briefly, 1 ㎎ freeze-dried samples dissolved in 95% methanol, and 1 ㎖ of extract solution, 10 ㎖ diethylene glycol and 0.1 ㎖ 1N NaOH were added to a 25 ㎖ volumetric flask. The mixture was incubated for 1 hour at 37°C in water bath. The absorbance was measured at 420 ㎚ using a spectrophotometer (Biochrom Co., England). Total flavonoid of the samples was expressed as mg narincin equivalent in 1 g dry weight of sample extract.
Assay of DPPH radical scavenging rate
One hundred ㎕ of various concentrations (1, 2.5, 5, 10 and 20 mg mL-1) of extracts in C. lanceolata were added to 900 ㎕ of 100% methanol containing 100 ㎛ DPPH, and the reaction mixture was shaken for 5 min in the slight vortex. Leaving room temperature for 30 min under darkness, the absorbance of DPPH was determined by spectrophotometer at 517 ㎚. The DPPH radical scavenging activity was calculated according to the following equation: Scavenging effect on DPPH radical (%) = [(A-B)/A]x100, Where A is the absorbance at 517 ㎚ without pigment compositions and B is the change in absorbance at 517 ㎚ with pigment compositions incubation (Brand-Williams et al., 1995).
Assay of cytotoxicity on human cancer cell lines
The cytotoxicity of C. lanceolata extracts was assayed using human cancer cell lines, including HeLa, Calu-6, and MCF-7 for human cervical carcinoma, pulmonary carcinoma, and breast adenocarcinoma, respectively. The cell lines were purchased from the Korean Cell Line Bank (KCLB) for the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The cells were plated on 96-well plates at a concentration of 3 × 104 cells/㎖. The cells were incubated for 24 h in RPMI-1640 medium at 37°C with 5% CO2 in a humidified incubator, and then treated with 2 ㎕ of various concentrations (50, 100, 200, 400, and 800 ㎍ ㎖-1) of the extracts. After incubation for 48 h, the cells were washed twice with phosphate buffer solution (PBS). The MTT solution (5 ㎎ ㎖-1) was dissolved in 1 ㎖ of PBS, and 10 ㎕ of this solution was added to each well. After a reaction period of 4 h, the solution in each well containing media, unbound MTT, and dead cells were removed by suction and 100 ㎕ of DMSO was added to each well. The plates were shaken for 15 min using a plate shaker, and the absorbance was recorded using an enzyme-linked immunosorbent assay (ELISA) reader (Bio-Rad model 550, USA) at a wavelength of 540 ㎚. Cell viability was determined as the percent of the viability of treated cells compared with that of the untreated cell, and the values were then used to iteratively calculate the concentration of extract required to induce a 50% reduction (IC50) in the growth of each cell line.
Assay of cytotoxicity on 3T3-L1 cell
The cytotoxicity of the samples was determined by using MTT assay. Cells were incubated for two days after seeded at a density of 1 x 105 cells/well on a 96-well plate. Then cells were incubated for another two days after treated with samples (n-Hexane, Methylene Chloride, Ethyl acetate, n-Butyl alcohol, Distilled water-soluble fraction). Next, 0.5 ㎎ ㎖-1 MTT solution was added into each well, and incubated for 3 hours at 37°C. The liquid in the plate was removed, and DMSO solution was added to dissolve the formazan complex completely. Absorbance was then measured at 520 ㎚ using an ELISA reader (Bio-Rad model 550, USA). Cell viability was calculated as following equation:
% Cell viability = (Absorbance in the presence of sample/ Absorbance of the control reation) x 100
Data analysis
The statistical analysis was performed using the procedures of the Statistical Analysis System (SAS version 9.1). The ANOVA procedure followed by Duncan test was used to determine the significant difference (p < 0.05) between treatment means.
Results and Discussion
Total polyphenol and flavonoid contents
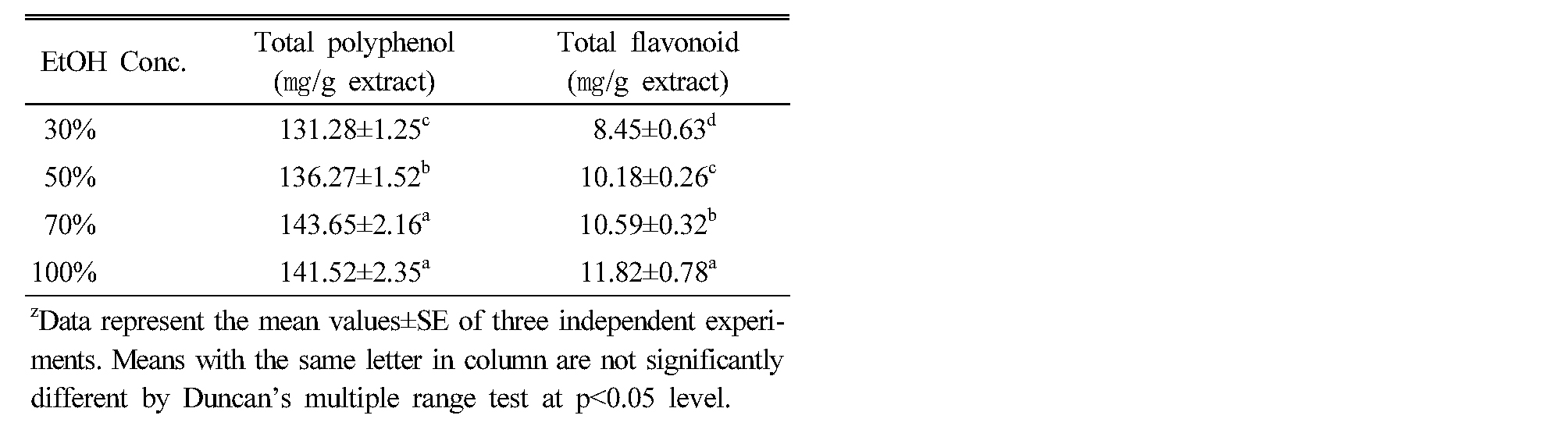
A number of studies have focused on the biological activities of phenolic compounds, which are potential antioxidants and free radical scavengers (Rice-Evans et al., 1995; Marja et al., 1999; Sugihara et al., 1999). Polyphenols are widely distributed in plants and phenolic antioxidants have been found to act as free radical scavengers as well as metal chelators (Shahidi and Wanasundara, 1992; Sanchez-Moreno et al., 1999). Total polyphenol content showed the highest amount in 70% ethanol extract (143.65 ㎎ g-1), and followed by 100% extract (141.52 ㎎ g-1), 50% extract (136.27 mg g-1) and 30% extract (131.28 ㎎ g-1). And the total flavonoid content showed the highest amount in 100% ethanol extract (11.82 ㎎ g-1), and followed by 70% extract (10.59 ㎎ g-1) and 50% extract (10.18 ㎎ g-1). However, 30% extract (8.45 ㎎ g-1) was the lowest (Table 1). The result was considerably consistent with the finding of DPPH radical scavenging activity (Velioglu et al., 1998). Zhou and Yu (2006) also reported that total phenolic content of the tested vegetable extracts was correlated with the DPPH radical scavenging activity, suggesting that total phenolics can play a major role in the antioxidant activity of plant materials.
Table 1. Total polyphenol and flavonoid contents according to the concentration of ethanol solvent in Codonopsis lanceolata |
|
DPPH radical scavenging activity
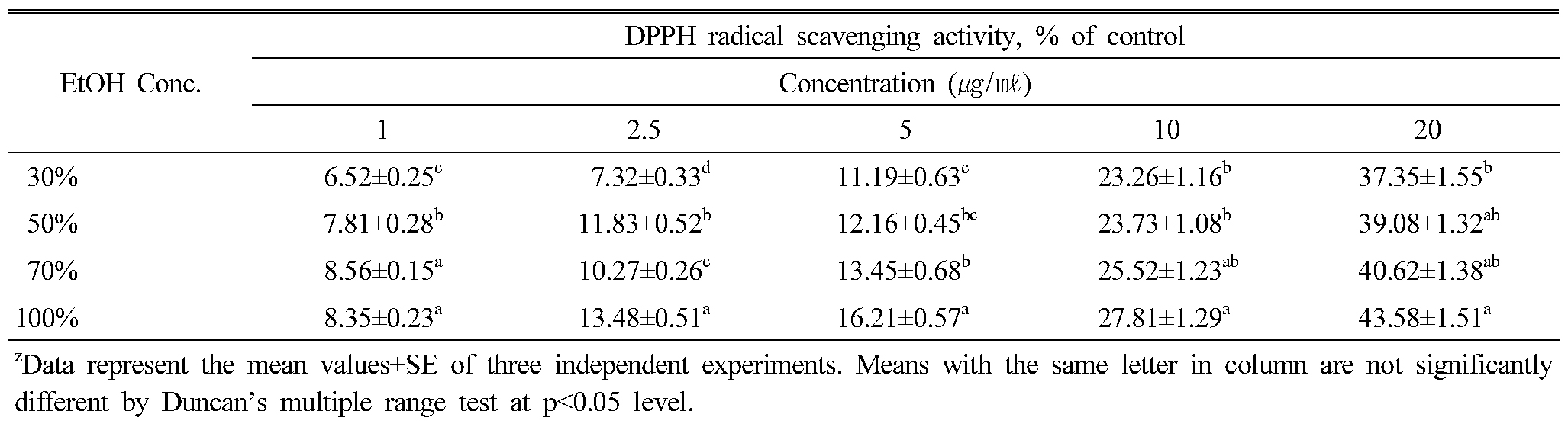
The extract of 100% ethanol solvent had the highest DPPH radical scavenging activity, and followed by 70% extract, 50% extract and 30% extract. Overall, the DPPH radical scavenging activity showed that the increase was proportional to the concentration (Table 2). However, there was no significant difference according to the solvent concentration. The investigation of the antioxidant activity of natural substances is based on the measuring of the electron donor capacity of DPPH with the ability to inhibit the oxidation by donating electrons in free radicals causing this lipid peroxidation (Boo et al., 2012), that is, free radical are known to be a major factor in biological damages, and DPPH has been used to evaluate the free radical-scavenging activity of natural antioxidants (Yokozawa et al., 1998; Zhu et al., 2001; Kim et al., 2017). Active oxygen caused by in vivo metabolism removed by the body's antioxidant system, but excessive free radicals induced stress, causing the lipid peroxidation by combining with unsaturated fatty acids in the cell membrane, and brought intracellular structural and functional damage. Cells are oxidized and damaged by the free radical, depending on the growth of cells. It has been reported that saponin components of P. grandiflorum have the antioxidant capacity to inhibit the oxidation by donating electrons to the free radical due to strong reduction (Fu et al., 2009; Kim et al., 2010; Ryu et al., 2012). The effective source of C. lanceolata could be employed in all medicinal preparation to combat myriad diseases associated with oxidative stress. In conclusion, ethanol extracts from C. lanceolata root showed high antioxidant activity, through measurement of DPPH free radical scavenging activity. The extracts of 70%, 100% ethanol showed high DPPH free radical scavenging activity with high levels of total phenolics and flavonoid. The medicinal plants increased the biological activities, in vitro in a dose-dependent manner. Results also showed that total phenolics level was highly related to the free radical scavenging activity.
Table 2. DPPH radical scavenging activities according to the concentration of ethanol solvent in Codonopsis lanceolata |
|
Cytotoxicity on human cancer cell
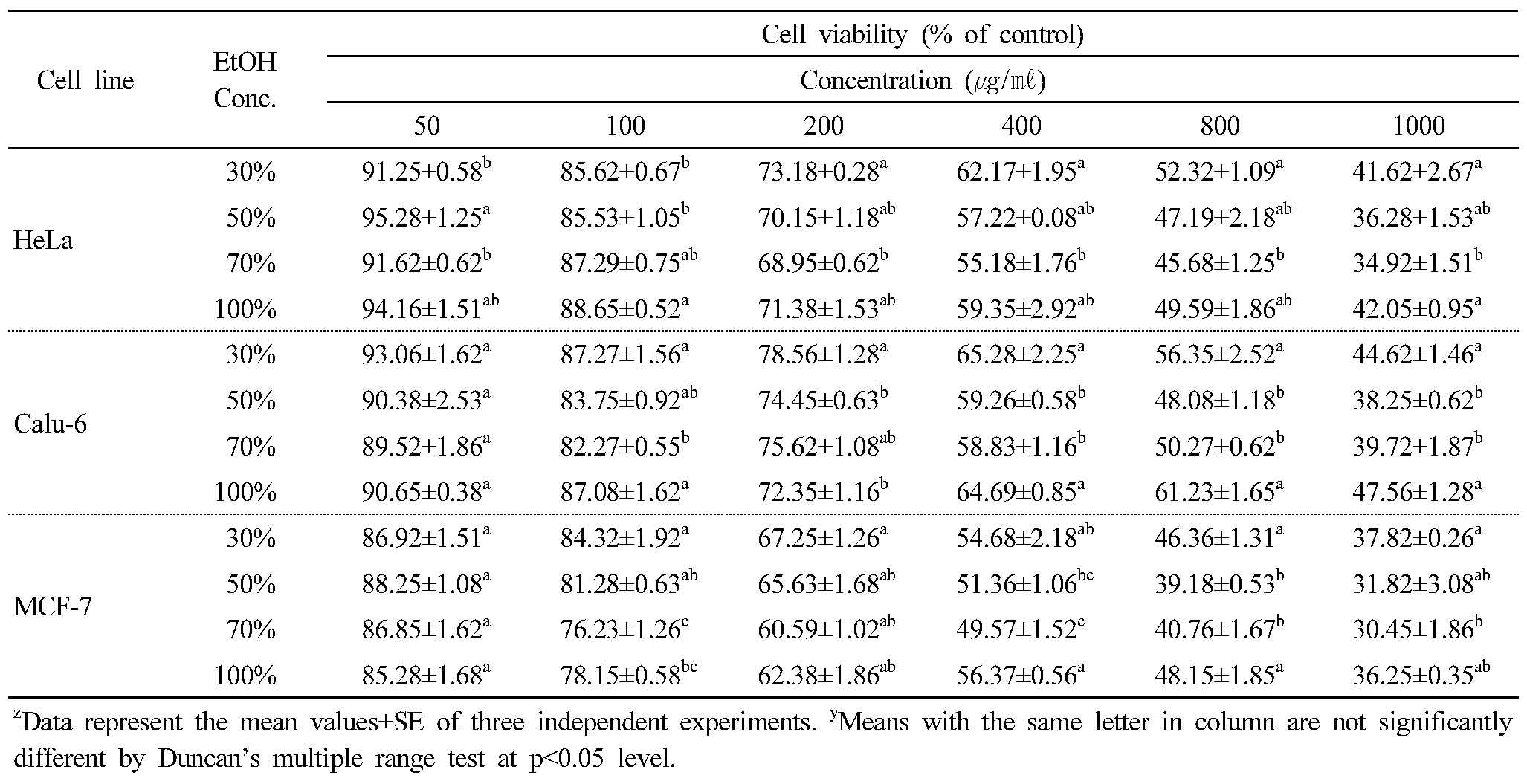
The cytotoxicity of C. lanceolata on three human cancer cell lines were evaluated by the MTT assay. When cells were treated for 48 hrs with various concentrations (50, 100, 200, 400, 800 and 1,000 ㎍ ㎖-1) of 30%, 50%, 70% and 100% ethanol extracts, the rate of cell survival progressively decreased in a dose-dependent manner. Results of the cytotoxicity evaluation against human cancer cell lines from roots of C. lanceolata are shown in Table 3 and Figure 1. Overall, the cytotoxicity of extracts from C. lanceolata exhibited significant differences in different ethanol solvent concentrations. That is, the cytotoxic effect against human cancer cell was higher in 50% and 70% ethanol extracts. In particular, the cytotoxic effect in MCF-7 cell was relatively higher than that of other cells (Table 3). The IC50 (concentration causing 50% cell death) value showed the highest on MCF-7 cell (538.39 ㎍ ㎖-1) in 70% ethanol extract, and exhibited significant activity against Hela cell (637.87 ㎍ ㎖-1), Calu-6 cell (728.64 ㎍ ㎖-1). The extracts of 50%, 70% ethanol showed high cytotoxic effect, especially, the cytotoxicity in MCF-7 cell showed higher than in other cells (Fig. 1). The persistency search for new anticancer compounds in plant medicine and traditional foods is a realistic and promising strategy for its prevention. Numerous compounds found in plants with anticancer properties are such as alkaloids, phenylpropanoids, and terpenoids (Kintzios, 2006; Park et al., 2008; Yan-Wei et al., 2009; Vijayarathna and Sasidharan, 2012). Presently there is an increasing interest world wide in herbal medicines accompanied by increased laboratory investigation into the pharmacological properties of the bioactive ingredients and their ability to treat various diseases (Lobo et al., 2009). It is well known that chemicals and medicinal plant medicines may produce toxic effects. Based on results presented in this paper, C. lanceolata can be used as a source of cytotoxic agent.
Table 3. Cytotoxicity of Codonopsis lanceolata extracts according to the concentration of ethanol solvent on three human cancer cell lines |
|
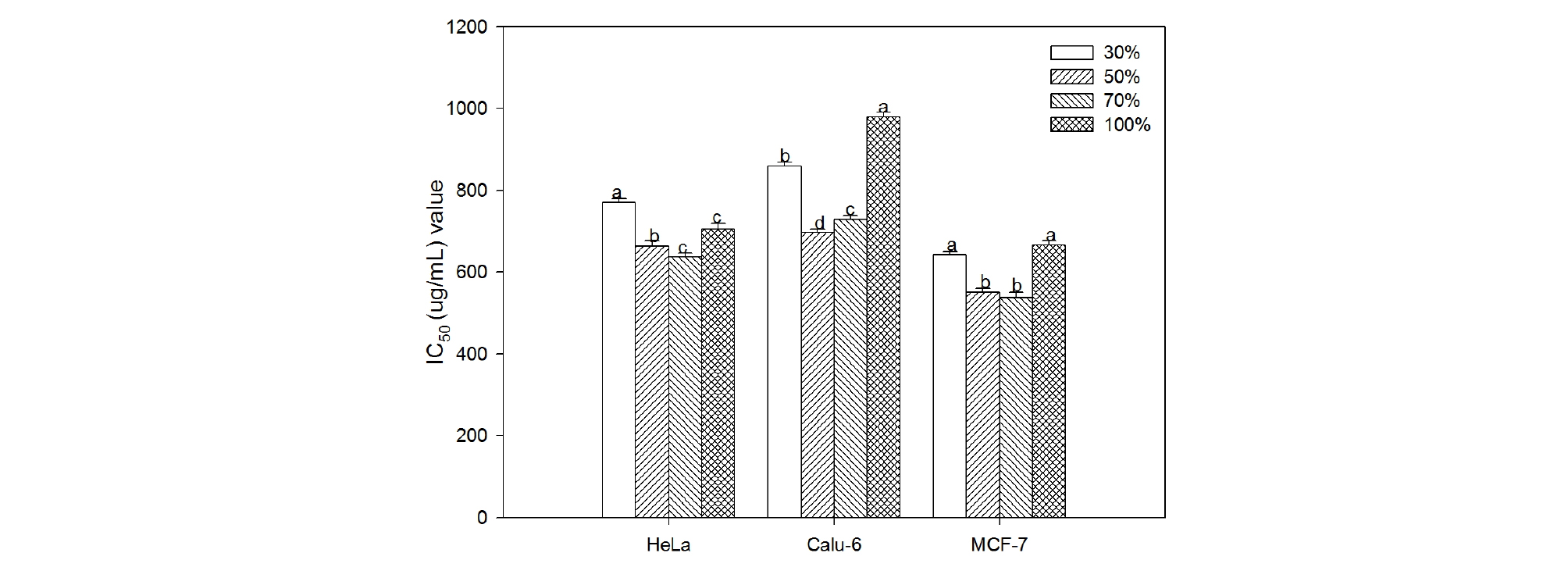
Fig. 1.
Cytotoxic activity (IC50 values) of Codonopsis lanceolata extracts according to the concentration of ethanol solvent on three human cancer cell lines. IC50 is defined as the extract concentration at which there was a 50% decrease in cell number. Each bar represent mean±SE of three experiments in duplicate. Means followed by the same letter are not significantly different at p < 0.05.
Cytotoxicity on 3T3-L1 cell
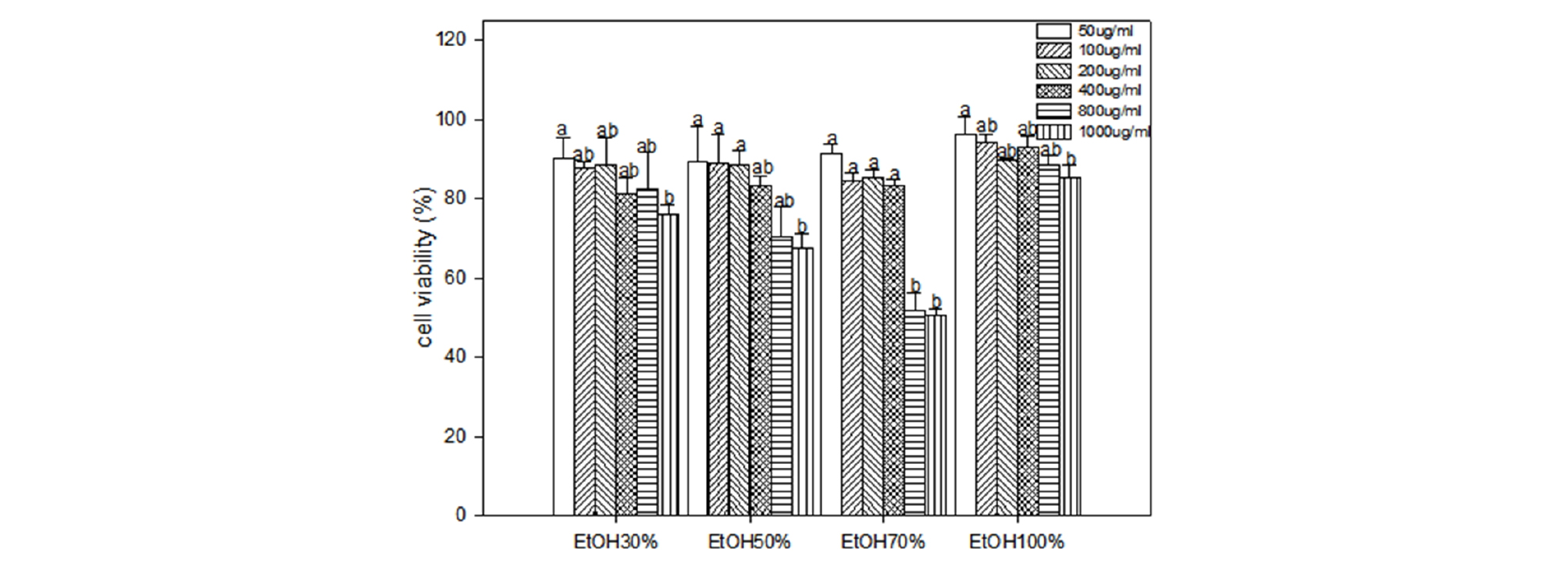
The cytotoxicity of C. lanceolata on 3T3-L1 preadipocytes were evaluated using the MTT assay. When cells were treated for two days with various concentrations (50, 100, 200, 400, 800 and 1,000 ㎍ ㎖-1) of extracts of different ethanol solvent, the rate of cell survival progressively decreased in a dose-dependent manner. The cytotoxicity evaluation against 3T3-L1 preadipocytes of different ethanol extracts from roots of C. lanceolata is shown in Figure 2. The extract of 70% ethanol at 1,000 ㎍ ㎖-1 exhibited a pronounced cytotoxic effect on 3T3-L1 cell comparable to that of the other extracts, and followed by 50%, 30% and 100% extract at the same concentration. Development of obesity is characterized by the growth of adipose tissue mass. Like growing tumors, growth and expansion of adipose tissue require the formation of new blood vessels or angiogenesis to provide oxygen and nutrients to adipocytes, which are expanding in both size and numbers. Thus, one strategy to reduce adiposity would be the inhibition of angiogenesis along with reducing adipocyte numbers and fat content of adipocytes (Ejaz et al., 2009). In the present study, we postulated an exciting observations that the root of C. lanceolata has the ability of cytotoxic effect against 3T3-L1 preadipocyte cell, and these findings are consistent with values determined in vegetables and other herbs (Cao et al., 2007; Roh and Jung, 2012).