Introduction
Materials and Methods
Preparation of a combination of grapefruit and rosemary extracts (cG&Re)
Analysis of marker substances by HPLC
Reagents and antibodies
Cell culture
Determination of cell viability
Cell viability assay in UVB-irradiated HaCaT cells
UVB-irradiated skin photo-aging animal model
Western blot analysis
Statistical analysis
Results
Quantification of marker substances in cG&Re extract
Effect of cG&Re on HaCaT cell viability
Inhibitory effects of cG&Re on UVB-induced MMPs and NF-κB in HaCaT cells
Suppressive effects of UVB-induced MAPKs on cG&Re in HaCaT cells
Inhibitory effects of cG&Re on MMPs and NF-κB in UVB-irradiated dorsal mouse skin
Inhibitory effects of cG&Re on MAPKs in UVB-induced dorsal mouse skin
Discussion
Introduction
Skin photo-aging is due to numerous factors, including chronic exposure to ultraviolet (UV) radiation from sunlight. UV radiation can be divided into three categories: UVA (315–400 ㎚), UVB (280–315 ㎚), and UVC (100–280 ㎚). Among these categories, UVA and UVB can harm human health when they reach the earth’s surface (Rosenthal et al., 1986). Symptoms of skin photo-aging include dryness, hyperpigmentation, oxidative stress, and wrinkles (Fujii et al., 2013; Sun et al., 2015). UVB irradiation increases reactive oxygen species (ROS) in the skin and breaks down extracellular matrix (ECM) components, such as collagens, gelatins, elastic fibers, and glycosaminoglycans (Naylor et al., 2011; Wolfle et al., 2011).
According to previous studies, ROS, matrix metalloproteinase (MMP) expression, and collagen/elastin degradation in ECM increased by UVB irradiation in skin tissue (Pittayapruek et al., 2016; Quan et al., 2009). Also, UVB promotes activation of signal cascades related to skin-aging, such as mitogen-activated protein kinases (MAPKs), including extracellular signal-regulated kinase (Erk), c-Jun N-terminal kinases (JNK), a class of MAPKs (P38), activated protein-1 (AP-1), and nuclear factor kappa B (NF-κB). Therefore, antioxidants that decrease ROS in natural materials can be used as agents to suppress skin damage (Afaq et al., 2006; Baek et al., 2016). MMPs have various subgroups. MMP-1, 8, and 13 are collagenases like native collagen-degradation factor, and MMP-2 and 9 are gelatinases that cleave the basement membrane. Conversely, MMP-3 and 10 are stromelysins that have more extensive substrate specificity than the previously mentioned subgroups (Davies, 2014). UV irradiation accelerates MMPs expression by various signal pathways, such as MAPKs, including Erk, P38, JNK, and NF-κB (Park et al., 2014; Rabe et al., 2006).
Grapefruit (Citrus paradisi) is a kind of citrus fruit that contains vitamins, minerals, and dietary fiber. The seeds and peel of grapefruits have an abundancy of antioxidants, including flavonoids, vitamin C, carotenoid, citric acid, and limonoid (Vanamala et al., 2006). Grapefruit seed extract has greater inhibitory activity against gram-positive than gram- negative bacteria (Park et al., 2006) and prevents inflammation- related diseases, such as cardiovascular disease, cancer, and diabetes (Jun et al., 2012).
Rosemary (Rosmarinus officinalis) from the Lamiaceae family is native to the Mediterranean region and has been used in many culinary applications and in medicine. Rosemary extract primarily contains polyphenols such as carnosic acid (CA) and rosmarinic acid (RA) (Cuvelier et al., 1994). Also, rosemary extract and its polyphenols, CA and RA, were recently found to have powerful anticancer effects (González-Vallinas et al., 2015; Moore et al., 2016; Petiwala et al., 2013).
A combination of natural plant extracts has more benefit than a single extract or compound (Chanda et al., 2011; Zhou et al., 2016). According to previous studies, the rosemary and citrus mixture extracts (cG&Re) showed that it may inhibit the incidence of skin photocarcinogenesis (Ham et al., 2015). Also, cG&Re indicated that it has decreased the elastase activity, skin thickness and suppressed skin erythema, transepidermal water loss (TEWL) compared to that in the UVB-irradiated group (Choi et al., 2019). However, specific molecular mechanisms of these extracts are clearly unknown.
In this study, skin aging-related biomarkers of specific MMPs, NF-κB, and MAPKs in HaCaT cell and skin tissues induced UVB-exposed damage were analyzed to investigate the molecular mechanisms underpinning the physiologic and regulatory roles of this cG&Re.
Materials and Methods
Preparation of a combination of grapefruit and rosemary extracts (cG&Re)
The herbal extract was a combination of grapefruit and rosemary extracts supplied by Monteloeder, S.L. (Miguel Servet 16, Elche, Alicante, Spain). The first standard of sample No.000001 of both raw materials is stored in Monteloeder, S.L. The herbal extract was identified by visual examination plus HPLC analysis. Dried rosemary (Rosmarinus officinalis) leaves were extracted with 70% Ethanol for 1 h at RT and grapefruits (Citrus paradisi) were extracted with 70% Ethanol for 2 h at 50°C and centrifuged separately and evaporated. The evaporated each extract was combined at a 1:1 ratio and analyzed by HPLC for standardization.
Analysis of marker substances by HPLC
As for a combination of grapefruit and rosemary extracts (cG&Re), marker substances were analyzed by HPLC. The 2 g of cG&Re extract were weighed accurately and placed into a 50 mL volumetric flask, and MeOH was added to a final volume of 50 mL and then sonicated for 30 min. This sample solution was then passed through a 0.45 ㎛ Nylon syringe filter and 20 μL was injected into HPLC. The marker substances were separated using Agilent HPLC system (a model G1312A binary LC pump, an auto sampler, and a diode-array detector). A C18 LiChrospher 100 column (250 x 4.6 ㎜ i.d., Merck, KGaA, Germany) was used to separate marker substances. The standards were purchased from Sigma Aldrich (St. Louis., MO, USA). Peaks were detected at 240 ㎚. The compounds were eluted with a gradient mobile phase consisting of 2.5% acetic acid prepared in nanopure water (A) and 100% acetonitrile (B) with the following solvent gradient: 0 min, 95% A; 15 min, 80% A; 25 min, 75% A; 40 min, 50% A; 70 min, 20% A; 80 min, 95% A. The contents of each reference in cG&Re extracts were determined using the calibration curve of marker substances.
Reagents and antibodies
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) solution was obtained from Sigma-Aldrich (St. Louis, MO, USA). Antibodies against matrix metalloproteinase (MMP-1, 8, and 13) were purchased from Abcam (Cambridge, UK), and antibodies against MMP-2 was obtained from GeneTex (Irvine, CA, USA). Antibodies against phospho-extracellular signal-regulated kinases (p-Erk), c-Jun N-terminal kinases (p-JNK), a class of MAPKs (p-P38), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), and β-actin were purchased from Bioworld Technology, Inc. (St. Louis Park, MN, USA).
Cell culture
HaCaT cells, the immortalized human keratinocyte cell line, were purchased from AddexBio Technologies (San Diego, CA, USA) and cultured in Dulbecco’s modified Eagle medium (DMEM) containing heat-inactivated 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (P/S) at 37°C in a humidified 5% carbon dioxide and 95% air incubator. The culture medium was routinely changed every 2 days.
Determination of cell viability
HaCaT cells were seeded in a 96-well plate at a density of 1 x 105 cells/mL and cultured at 37°C in a humidified incubator with 5% carbon dioxide and 95% air for 24 h. A cG&Re combination was added in a dose-dependent manner (0, 10, 25, 50, and 100 ㎍/mL) and incubated at 37°C in 5% carbon dioxide for 24 h. To evaluate cell viability, the MTT solution (final concentration 5 ㎎/mL) was added and cells were incubated at 37°C in 5% carbon dioxide for an additional 1 h. Then, removing the culture medium, including MTT solution, 100 μL of dimethylsulfoxide (DMSO) per well was added to the cells to dissolve formazan and measured using a microplate reader at 595 ㎚ (Victor3; Perkin Elmer, Waltham, MA, USA). The control-group untreated sample was considered as 100% viable cells. Results were expressed as percent of viable cells compared with the control group.
Cell viability assay in UVB-irradiated HaCaT cells
Cell viability was determined using MTT solution. HaCaT cells at a concentration of 1 x 105 cells/mL in 200 μL of culture medium were seeded in a 96-well plate. Cells were incubated for 24 h at 37°C in a humidified incubator with 5% carbon dioxide and 95% air, and then dose-dependently (10, 25, 50 ㎍/mL) treated with a cG&Re combination in 200 μL of culture medium. Next, the culture medium was changed to 100 μL of phosphate-buffered saline (PBS) and the plates were exposed to 80 mJ/cm2 of UVB using a UV lamp (Bio-Link crosslinker; Analis, Suarlée, Belgium). After UVB irradiation, cells were re-treated with the same concentrations of cG&Re. After cell medium was removed, MTT solution (final concentration 5 ㎎/mL) was added to fresh medium and incubated for 1 h at 37°C and with 5% carbon dioxide. Then, the culture medium, including MTT solution, was removed and 100 μL of DMSO per well was added to the cells to dissolve formazan. The absorbance of samples was measured using a microplate reader at 595 ㎚ (Victor3; Perkin Elmer). Epigallocatechin gallate (EGCG 50 ㎍/mL) was used as a positive control in this study.
UVB-irradiated skin photo-aging animal model
Male BALB/c mice (aged 6 weeks) were obtained from Samtako (Osan-si, Gyeonggi-do, Republic of Korea) and housed under 12 h light/12 h dark cycles in a temperature- and humidity-controlled room (22 ± 1°C at 50% relative humidity). In this study, all mice were handled in accordance with the Laboratory Animal Ethics Committee (KNU 2018-0073), Kyungpook National University (Daegu, Republic of Korea), and were divided into five groups (n=5 per group): vehicle-treated (distilled water, group I); UVB irradiation only (distilled water, group II); UVB irradiation with oral EGCG 10 ㎎/day/㎏ (group III); UVB irradiation with oral cG&Re 10 ㎎/day/㎏ (group IV); and UVB irradiation with oral cG&Re 50 ㎎/day/㎏ (group V).
cG&Re was dissolved in an appropriate amount of distilled water before use. Samples were orally administered to mice, who were then exposed to 75 mJ/cm2 of UVB using a UV lamp for 2 weeks, with a gradual increase from 1 to 3 minimal erythema response (MED). EGCG was used as the positive control in this study.
Western blot analysis
HaCaT cells were seeded in 6-well plates (0.3 x 106 cells/mL) and incubated with DMEM including heat-inactivated 10% FBS, and 1% P/S. After incubating for 24 h, cells were pre-treated with cG&Re for 24 h. Then, the plates were exposed to 80 mJ/cm2 of UVB using a UV lamp, and media were removed. UVB-exposed dorsal skin tissues were obtained from sacrificed mice and, after harvesting, it was immediately stored in a deep-freezer and used. The cells and homogenized dorsal skin tissues of mice were lysed with radioimmunoprecipitation assay (RIPA) buffer (ELPIS Biotech, Inc., Daejeon, Republic of Korea), including a protease-inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA) and a phosphatase-inhibitor cocktail (Sigma-Aldrich). The lysates were centrifuged at 12,000 rpm for 30 min at 4℃ and protein concentrations were measured using the PierceTM BCA protein assay kit (Thermo Fisher Scientific, Waltham, MA, USA). The proteins were separated on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto a nitrocellulose membrane (Whatman, Dassel, Lower Saxony, Germany).
The membranes were blocked with 5% skim milk and 1% bovine serum albumin in Tris-buffered saline (TBS) containing 0.1% Tween 20 (TBST) for 1 h at room temperature and were then incubated with primary antibodies at 4℃ for 16-18 h. The membranes were then probed with anti-mouse, rabbit, goat immunoglobulin G-horseradish peroxidase-conjugated secondary antibody (Bethyl Laboratories Inc., Montgomery, TX, USA) at room temperature for 2 h. The immunoreactive bands were visualized using enhanced chemiluminescence reagents (ChemiDoc™ XRS+; Bio-Rad, Hercules, Contra Costa County, CA, USA). One representative blot from 3 independent experiments. The values indicated under each blot are the mean fold (%) protein expression relative to controls taken as 1 after normalization by β-actin (Image J quantification).
Statistical analysis
All experiments were repeated at least three times, and data were expressed as mean ± standard deviation (SD). Group means were compared using the non-parametric Kruskal- Wallis and Mann-Whitney tests with Statistical Package for the Social Sciences (SPSS Inc., Armonk, NY, USA); significance was tested at p < 0.01 and p < 0.05.
Results
Quantification of marker substances in cG&Re extract
We selected the marker substances naringin is as for grapefruit and carnosic acid and carnosol are as for rosemary. Carnosic acid is a major phenolic compound of rosemary. Several researchers have reported the degradation mechanism of carnosic acid to carnosol (Grace et al., 2017; Xiaojuan et al., 2013; Zhang et al., 2012). It is necessary to consider the specifications by the sum of the carnosic acid plus the carnosol for quality control.
The HPLC profile of cG&Re extract was shown in Fig. 1A. The contents of marker substances were determined using calibration curve. The calibration curves of naringin, carnosic acid and carnosol were constructed using each standard reagent, respectively (Table 1). In cG&Re extract, naringin was 20.37% the carnosic acid was 2.71% and carnosol content was 0.95% on a dry weight basis.
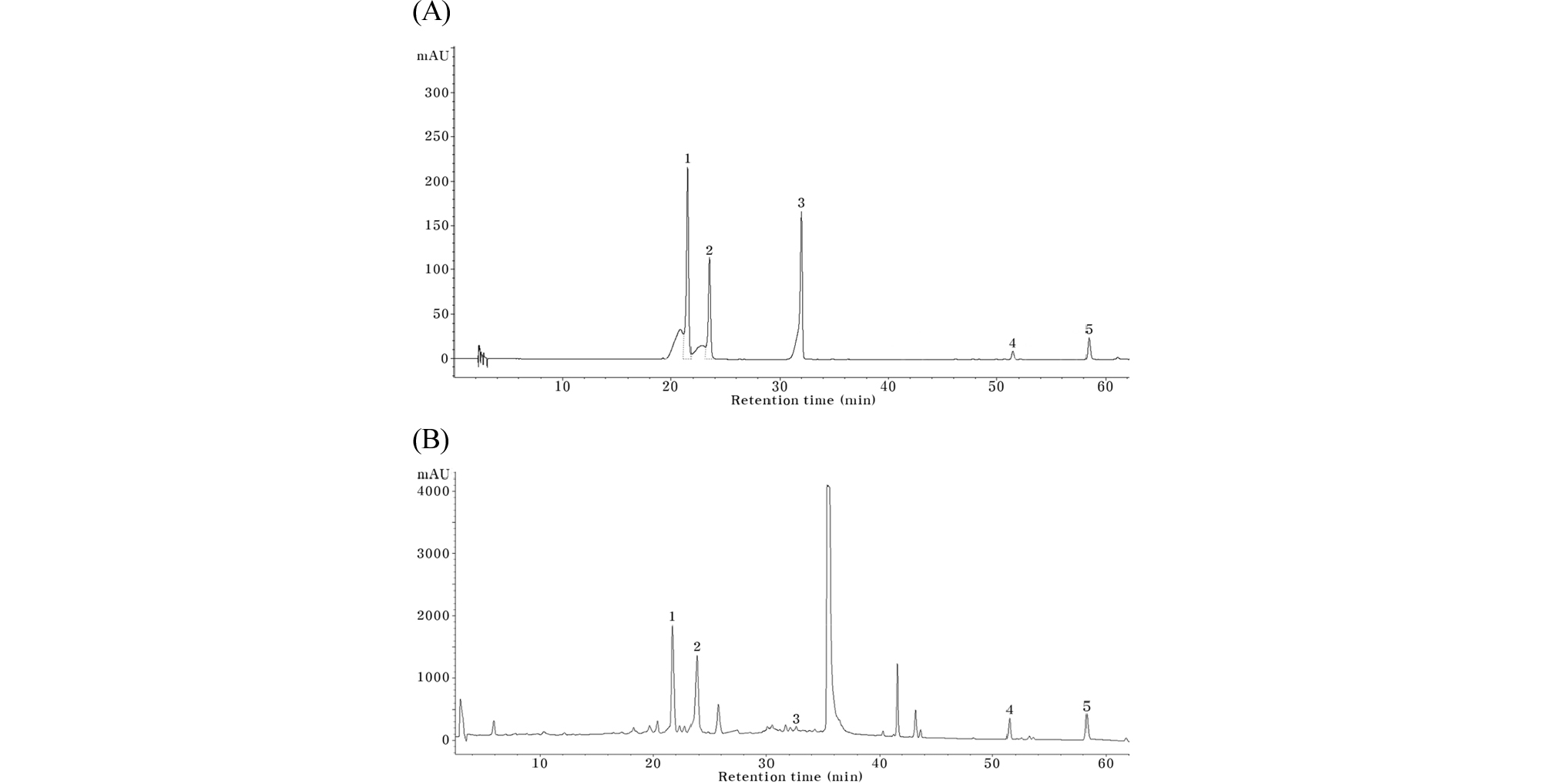
Fig. 1.
High-performance liquid chromatography analyses of cG&Re extract and mixture of NA, RA, PR, CL and CA. (A) and (B) are analysis for cG&Re extract and for mixture of NA, RA, PR, CL and CA under gradient elution system, respectively. The chromatographs were recorded at 240 ㎚. 1. NA, naringin, 2. RA, rosmarinic acid, 3. poncirin, 4. CL, carnosol, 5. CA, carnosic acid.
Table 1. Contents of marker substances in the cG&Re extracts
| Compounds | Contents (dry basis, %) | Calibration curve | R2 |
| Naringin | 20.37 ± 0.21 | y=21.403x - 35.54 | 0.9999 |
| Carnosic acid | 2.71 ± 0.01 | y=32.862x - 81.32 | 0.9999 |
| Carnosol | 0.95 ± 0.02 | y=27.837x + 0.669 | 0.9996 |
Effect of cG&Re on HaCaT cell viability
To investigate the viability of HaCaT cells in the presence of cG&Re, cells were incubated for 24 h with the extracts at 10, 25, 50, and 100 ㎍/mL concentrations. In the results, the cell viability was above 80% up to 50 ㎍/mL of cG&Re concentrations (Fig. 2A). To determine the extent of cell damage by UVB irradiation, cells were exposed to 40, 60, 80, and 100 mJ/cm2 and incubated for 12 h. Cell damage increased gradually depending on UVB dose and further experiments were performed at 80 mJ/cm2, which showed about 50% cell damage (Fig. 2B). Importantly, cG&Re 10, 25, and 50 ㎍/mL dose-dependently protected the HaCaT cell by suppressing UVB-induced cell death. 50 ㎍/mL of cG&Re had a similar cell-protective effect to EGCG used as a positive control (Fig. 2C).
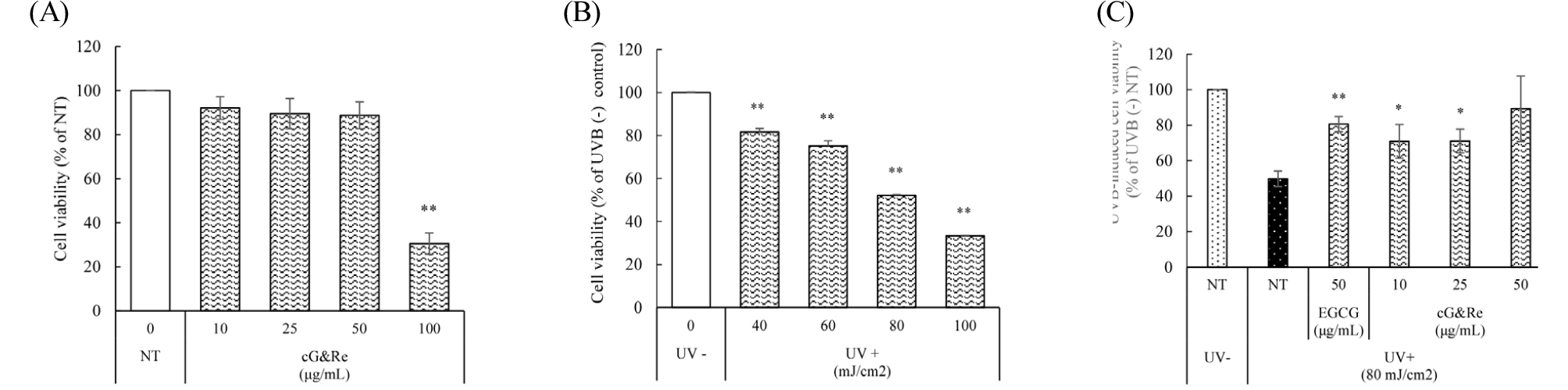
Fig. 2.
Effect of cG&Re treatment and UVB irradiation on HaCaT cell viability. (A) HaCaT cells were treated with dose-dependent concentrations of cG&Re for 24 h without UVB (B) HaCaT cells were exposed to UVB radiation (40, 60, 80, 100 mJ/cm2). (C) After the cells were pre-treated with cG&Re for 24 h, they were irradiated with 80 mJ/cm2 UVB. The cell viability was determined using the MTT assay. EGCG was used as the positive control. All data were presented as the mean ± SD of three independent experiments. *: p < 0.05, **: p < 0.01 compared with UV (-) NT (Non-treatment).
Inhibitory effects of cG&Re on UVB-induced MMPs and NF-κB in HaCaT cells
In HaCaT cells, UVB irradiation induced the expression of MMPs, which play important roles in the degradation of ECM components during skin photo-aging (Brenneisen et al., 2002; Chung et al., 2003; Rittie and Fisher, 2002), and accelerated the effects of NF-κB expression, a nuclear transcription factor, on UVB-induced MMP expression (Bond et al., 1999). UVB increased the levels of MMP-1, 2, and 8, whereas cG&Re pretreatment with 10, 25, and 50 ㎍/mL concentration significantly suppressed such MMPs expression in a dose-dependent manner. Moreover, cG&Re inhibited NF-κB expression activated by UVB irradiation (Fig. 3A and B). 50 ㎍/mL of cG&Re similarly reduced the expression of each protein, compared to EGCG. These results indicate that cG&Re is a potentially related to inhibition of UVB-induced expression of MMP-1, 2, and 8, and NF-κB in HaCaT cells.
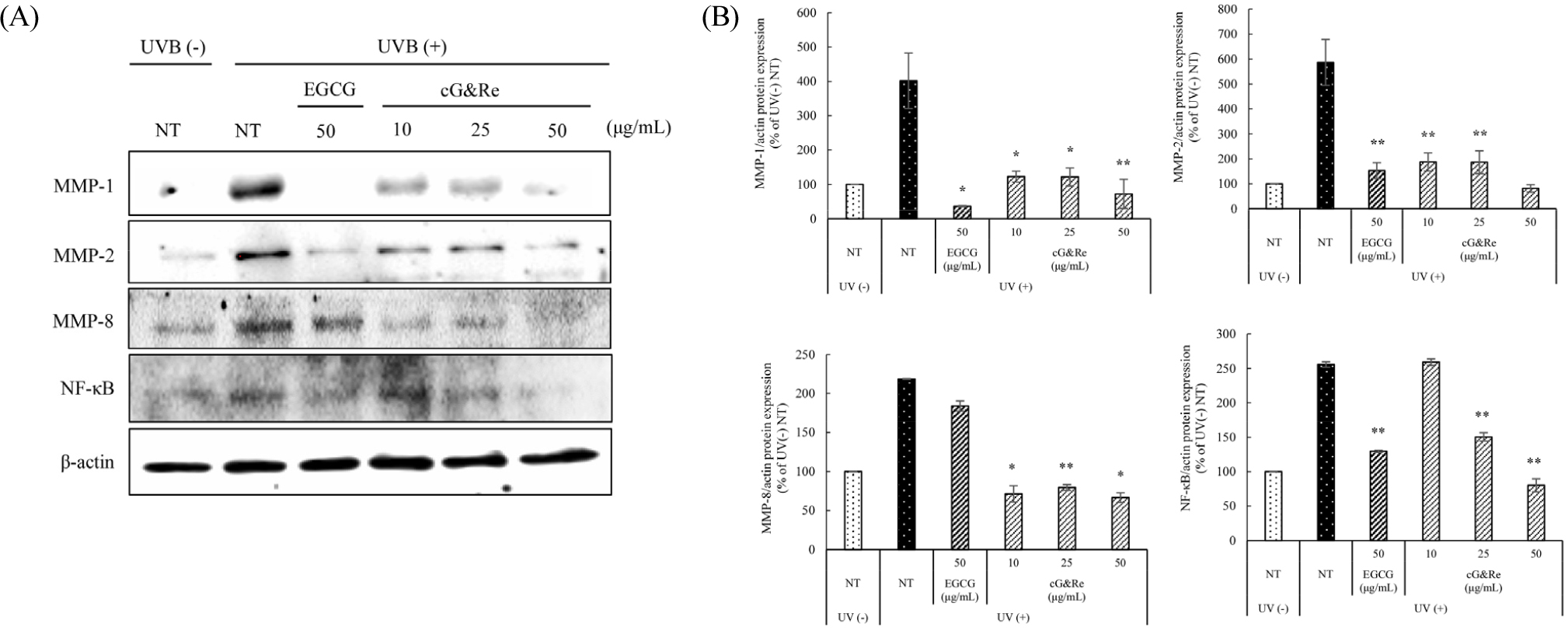
Fig. 3.
Inhibitory effects of cG&Re treatment on UVB-induced MMP-1, 2, 8, and NF-κB protein expression in HaCaT cells. HaCaT cells were pre-treated with different concentrations of cG&Re for 24 h, and then irradiated with 80 mJ/cm2 UVB. (A) MMPs (MMP-1, 2, 8) and NF-κB protein expression was determined using western blot. (B) Data were presented as mean ± SD of three independent experiments. *: p < 0.05, **: p < 0.01 compared with untreated control. All values (%) of MMP-1, 2, 8, and NF-κB were normalized by β-actin and quantified using Image J program.
Suppressive effects of UVB-induced MAPKs on cG&Re in HaCaT cells
MAPKs including Erk, JNK, and P38 was reported to activate NF-κB (Doyle et al., 1997). For anti-photoaging, phosphorylation of MAPK pathway should be decreased. Shown in Fig. 4A and B, pretreatment with cG&Re 10, 25, and 50 ㎍/mL remarkably decreased phosphorylation of UVB induced Erk, JNK, and P38 kinases in a dose-dependent manner.
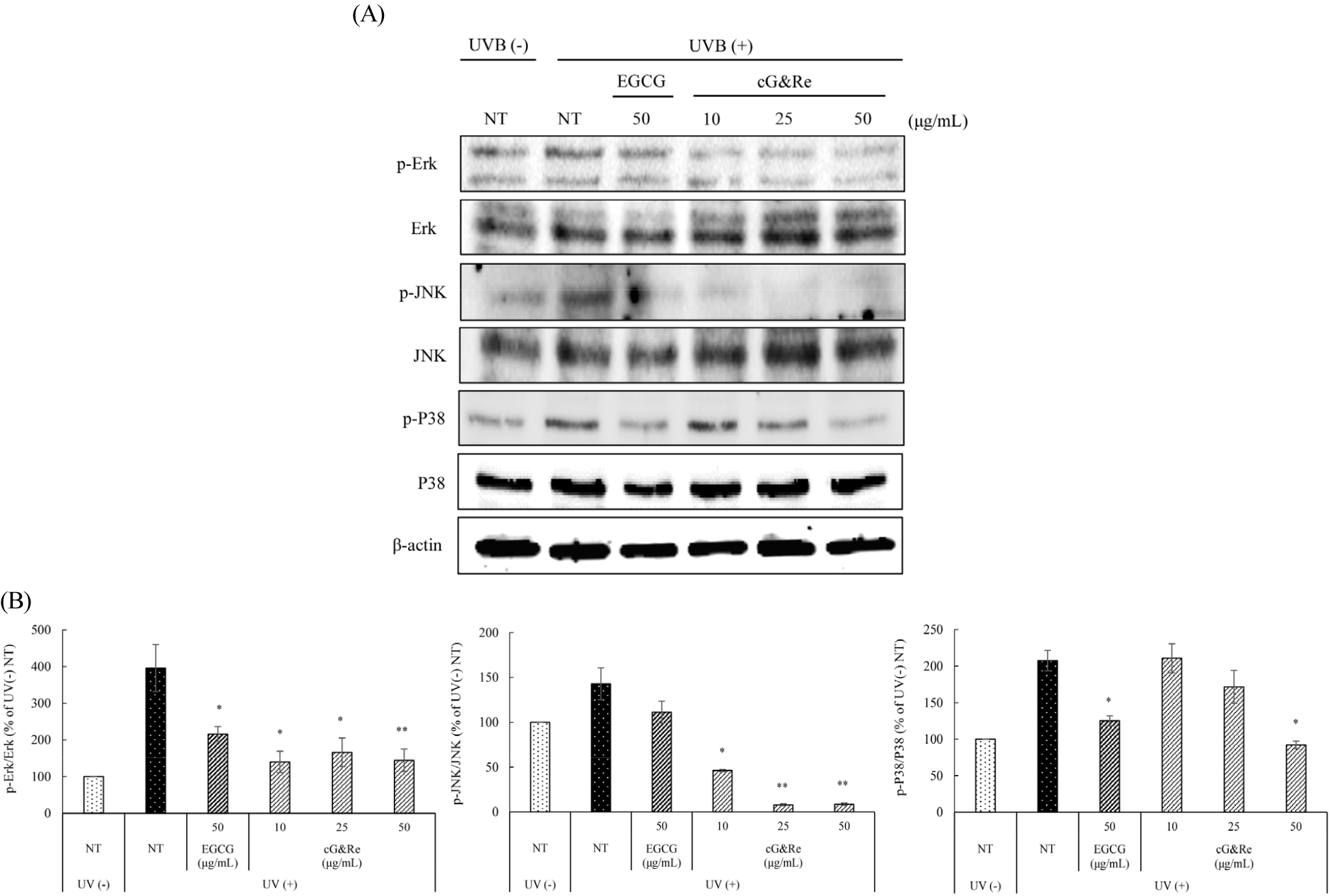
Fig. 4.
Inhibitory effects of cG&Re on UVB-induced p-MAPKs (p-Erk, p-JNK, p-P38) expression in HaCaT cells. HaCaT cells were pre-treated with different concentrations of cG&Re for 24 h, and then irradiated with 80 mJ/cm2 UVB. (A) p-MAPKs (p-Erk, p-JNK, p-P38) protein expression was determined using western blot. (B) Data were presented as mean ± SD of three independent experiments. *: p < 0.05, **: p < 0.01 compared with untreated control. All values (%) of phosphorylated Erk, JNK, and P38 were normalized by Erk, JNK, P38 and quantified using Image J program.
Inhibitory effects of cG&Re on MMPs and NF-κB in UVB-irradiated dorsal mouse skin
The effects of cG&Re on BALB/c mice was investigated and analyzed protein expression patterns of various factors, including MMP-1, 2, 13, and NF-κB, induced by UVB exposure. In Fig. 5A and B, UVB-induced MMP-1, 2, 13, and NF-κB expression had been significantly suppressed in cG&Re oral administration BALB/c mice group. These results in dorsal skin tissues were consistent with those seen in HaCaT cells. These results may suggest cG&Re inhibits skin damage by modulating protein expression of MMPs and NF-κB levels induced by UVB in skin tissues.
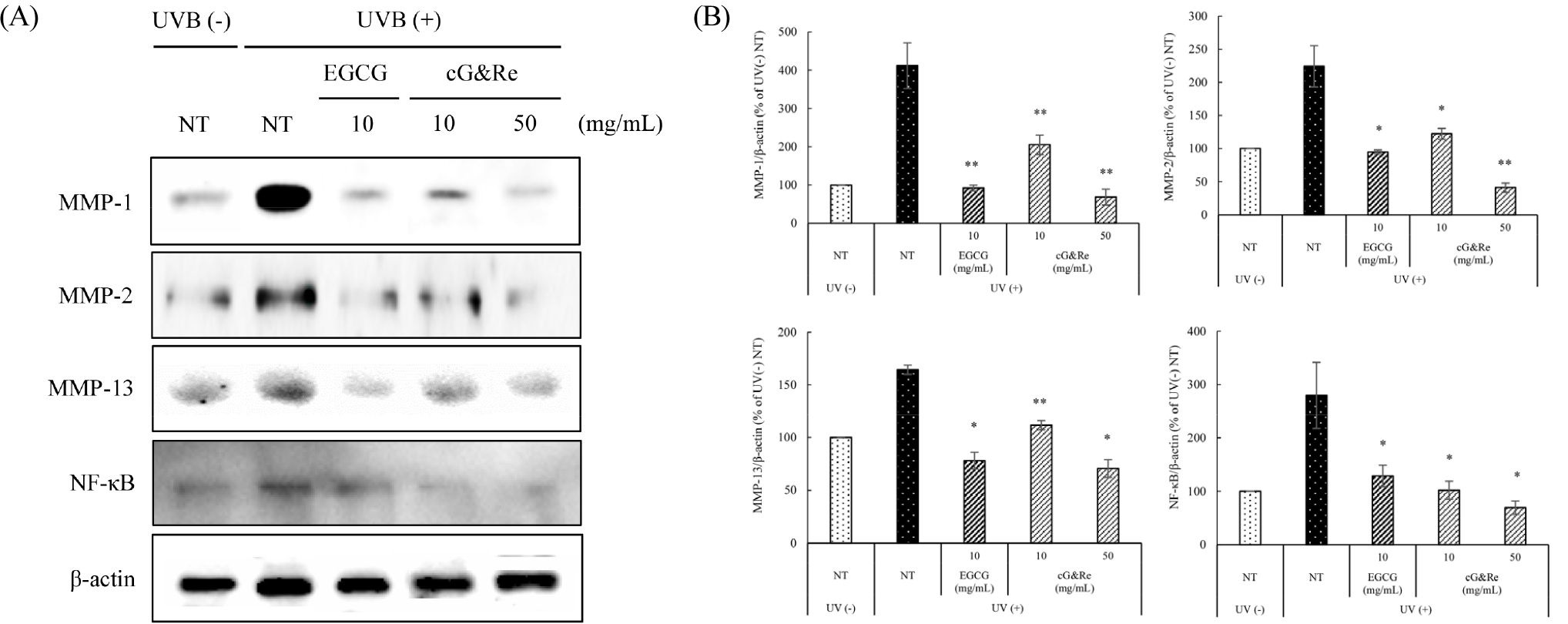
Fig. 5.
Inhibitory effects of cG&Re on MMP-1, 2, 13, and NF-κB protein expression in UVB-irradiated dorsal skin tissues in vivo. Mice were fed different concentrations of cG&Re extracts (10, 50 ㎎/day/㎏ (PO; per oral)) for 2 weeks and irradiated with 1MED (75 mJ/cm2) UVB for 1 min at mid time point. (A) MMPs (MMP-1, 2, 13), and NF-κB protein expression was determined using western blot. (B) Data were presented as mean ± SD of three independent experiments. *: p < 0.05, **: p < 0.01 compared with untreated control. All values (%) of MMP-1, 2, 8, and NF-κB were normalized by β-actin and quantified using Image J program.
Inhibitory effects of cG&Re on MAPKs in UVB-induced dorsal mouse skin
The MAPK-signaling pathway, an upstream regulator of MMPs, has a pivotal role in regulating cell proliferation, cell survival, and skin aging (Karin, 1995) and is known to be activated by UVB. As shown in Fig. 6A and B, phosphorylation of Erk, JNK, and P38 was remarkably increased by UVB irradiation, compared with the UVB non-irradiated group. Oral administration of cG&Re 10 and 50 ㎎/mL significantly inhibited the phosphorylation of MAPKs, such as Erk, JNK, and P38 in mice. These results indicate that orally administered cG&Re suppresses activated Erk, JNK, and P38 protein expression (induced by UVB irradiation in tissues) via regulation of the MAPK-signaling pathway.
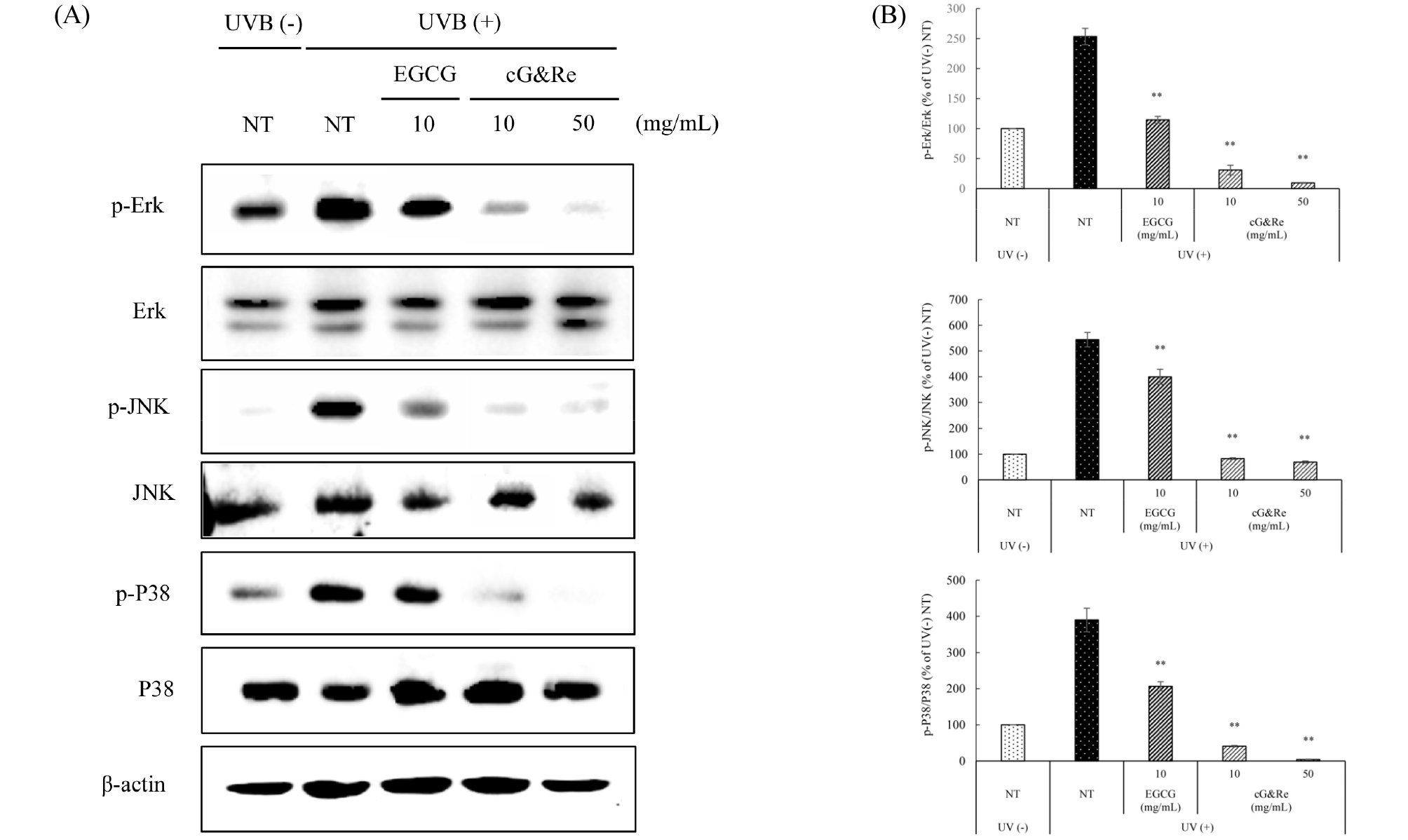
Fig. 6.
Inhibitory effects of cG&Re on UVB-induced p-MAPKs (p-Erk, p-JNK, p-P38) protein expression in UVB-irradiated dorsal skin tissues. Mice were fed different concentrations of cG&Re extracts (10, 50 ㎎/day/㎏ (PO; per oral)) for 2 weeks and irradiated with 1MED (75 mJ/cm2) UVB for 1 min at mid time point. (A) p-MAPKs (p-Erk, p-JNK, p-P38) protein expression was determined using western blot. (B) Data were presented as mean ± SD of three independent experiments. *: p < 0.05, **: p < 0.01 compared with untreated control. All values (%) of phosphorylated Erk, JNK, and P38 were normalized by Erk, JNK, P38 and quantified using Image J program.
Discussion
In the last decade, the levels of ultraviolet (UV) reaching the ground have increased by various environment change. Among these UV, UVB especially cause both direct and indirect skin damage and photoaging because of the increasing production of reactive oxygen species (ROS) and accelerating degradation of collagen/elastin in keratinocytes (Black, 2004; Jin et al., 2007; Kim et al., 2015). UVB exposure accelerates MMPs expression and phosphorylation of MAPKs, as well as NF-κB expression (Park et al., 2014; Rabe et al., 2006). Therefore, it means MMPs and MAPK phosphorylation suppression leads to inhibition of collagen and elastin degradation. Many studies have been reported to the development of anti-skin aging agent based on the mechanism of MMPs and MAPK phosphorylation suppression (Khan and Abourashed, 2011).
In this study, the anti-skin aging effects of a combination of grapefruit and rosemary extracts (cG&Re) was investigated in both UVB-irradiated in HaCaT cells and BALB/c mouse dorsal skin. First, the cell toxicity after treatment with cG&Re in dose-dependent manner (10, 25, 50, 100 ㎍/mL) did not appear up to 50 ㎍/mL (cell viability >80%) (Fig. 2A). We examined the protective effects of cG&Re on cell damage by UVB irradiation (80 mJ/cm2). Cell viability was dose- dependently increased by cG&Re treatment in UVB-exposed HaCaT cells.
UVB may induce inflammatory responses including the production of various inflammatory cytokines and chemokines, and the induction of cell death (Yoshizumi et al., 2008). It has been reported that cG&Re decreased the levels of IL-1β related to inflammation, a kind of cytokines, in skin tissues (Choi et al., 2019).
Additionally, we examined MMPs protein expression known to be related to collagen and elastin degradation in UVB-induced HaCaT cells and skin tissues. UVB-induced protein expression of MMP-1, 2, 8, 13 in HaCaT cells and dorsal skin tissues was suppressed by pre-treated cG&Re in dose-dependent manner (Figs. 3 and 5). These results are similar to previous report that mRNA and protein levels of MMP-1, 13 treated with cG&Re was decreased in UVB-exposed mouse (Choi et al., 2019).
The transcription of several MMPs was regulated by nuclear factor-κB (NF-κB), known as upstream regulator of MMPs proteins. (Ficher et al., 1996). In the results, cG&Re dose-dependently suppressed UVB-induced protein expression of NF-κB in HaCaT cells and dorsal skin tissues (Figs. 3 and 5).
Also, the inhibition of the MMP-2, 3, 9, and 13 expressions of by suppressing activities of MAPKs including JNK are associated with reduced wrinkle formation (Ficher et al., 1996). Our results show that cG&Re significantly inhibited the phosphorylation of MAPKs, such as Erk, JNK, and P38 in UVB-irradiated HaCaT cells and dorsal skin tissues (Figs. 4 and 6).
All results were shown that MMPs, MAPKs, and NF-κB expression was decreased by cG&Re treatment in dose- dependent manner. cG&Re could be helpful to suppress the collagen and elastin degradation and beneficial to prevent wrinkle formation and skin photo-aging induced by UVB exposure.
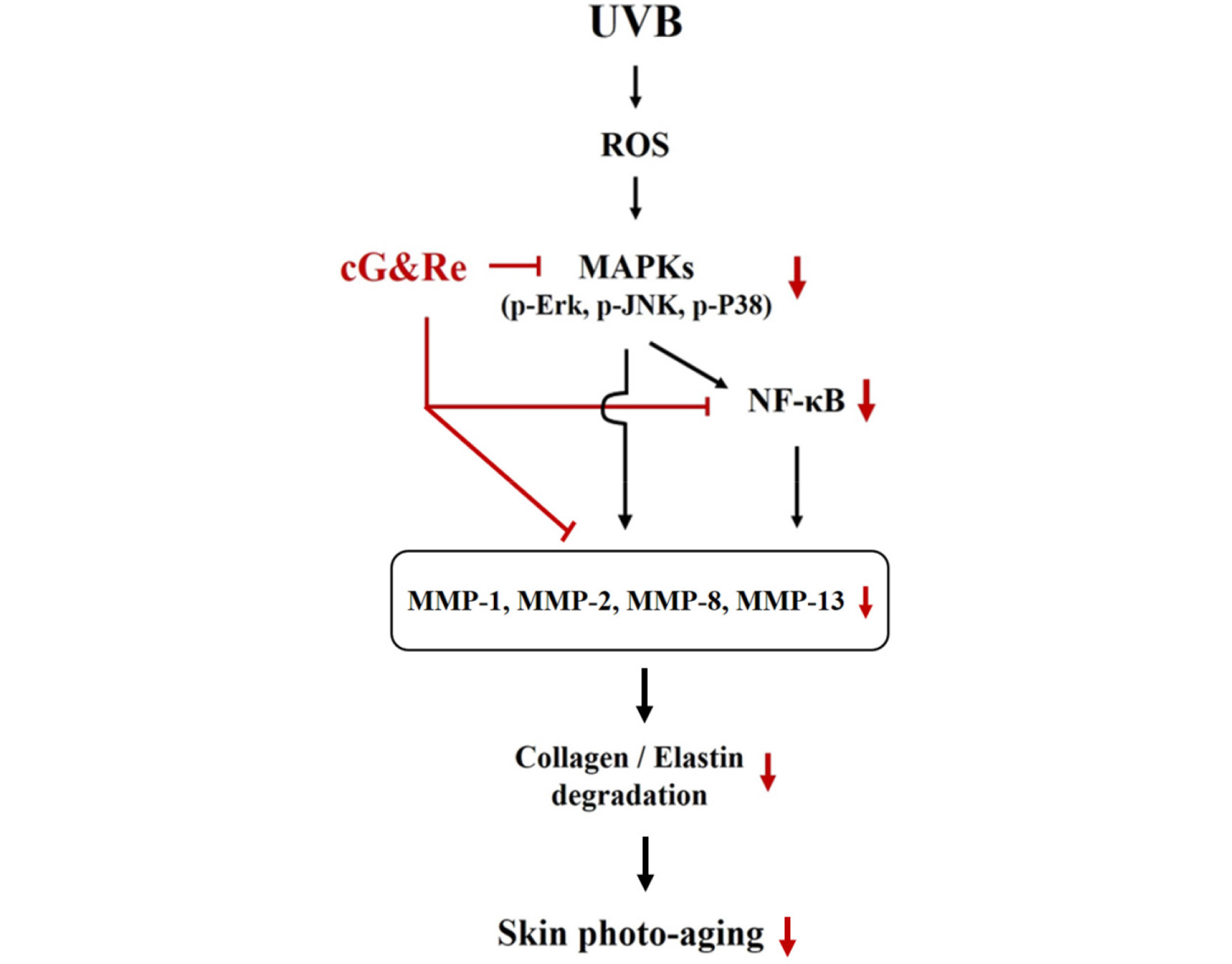
This study was aimed to investigate anti-skin aging effects of cG&Re through various signaling molecules in vitro and in vivo. A combination of grapefruit and rosemary extracts (cG&Re) dose-dependently inhibited cell toxicity induced by UVB irradiation in HaCaT cells. It is known that UVB accelerates MMPs, NF-κB and phosphorylation of MAPK signals. In HaCaT cell and BALB/c mice dorsal skin tissue, cG&Re inhibited acceleration of several MMPs and phosphorylation of P38, JNK and Erk in cytosol and NF-κB in nucleus (Fig. 7).
Taken all together, these results show that cG&Re inhibit expression of various MMPs (MMP-1, 2, 8, 13), NF-κB and MAPKs, upstream MMP regulators in cytoplasma. MMPs and MAPKs pathway phosphorylation suppression leads to inhibition of collagen and elastin degradation by UVB. It suggests that cG&Re may be a promising source for the development of functional supplement for preventing and delaying skin photo aging caused by chronic exposure to UVB from sunlight.